Quantum mechanics, or quantum physics, is one of the most profound and enigmatic fields in science. It governs the behavior of matter and energy on the smallest scales—the atomic and subatomic levels. Despite its complex and abstract nature, quantum mechanics has proven to be incredibly successful in explaining the universe’s fundamental workings. It has enabled the creation of technologies such as semiconductors, lasers, and MRI scanners, revolutionizing fields from electronics to medicine. Yet, for all its applications, quantum mechanics remains a source of confusion, awe, and wonder, both for scientists and the general public.
The Birth of Quantum Mechanics
Quantum mechanics emerged in the early 20th century as scientists began to encounter phenomena that classical physics could not explain. The need for a new theory arose particularly from the study of light and the behavior of atoms. Classical physics, as defined by Newtonian mechanics and electromagnetism, was not enough to account for the strange results obtained in experiments dealing with microscopic particles.
The story of quantum mechanics began in 1900 when German physicist Max Planck proposed a radical new theory to explain the behavior of blackbody radiation. Planck discovered that energy is emitted or absorbed in discrete quantities called “quanta,” rather than in a continuous flow as classical theory suggested. This idea of quantization was the first key step toward the development of quantum mechanics.
In 1905, Albert Einstein expanded on Planck’s work by explaining the photoelectric effect, where light striking a metal surface can cause electrons to be ejected from the metal. Einstein proposed that light itself is quantized into particles called photons, which are responsible for transferring energy to the electrons. This was a groundbreaking idea that challenged the classical wave theory of light.
As the years passed, more discoveries piled up that couldn’t be explained by classical physics. Niels Bohr developed a model of the atom in 1913 that incorporated the idea of quantized energy levels, and the quantum mechanical model of the atom gradually took shape. Then, in the 1920s, a series of brilliant scientists, including Werner Heisenberg, Erwin Schrödinger, and Paul Dirac, helped to form the foundation of quantum mechanics as we know it today.
The Fundamental Principles of Quantum Mechanics
Quantum mechanics is governed by a set of principles that differ significantly from those of classical physics. Some of the most important concepts include the following:
1. Wave-Particle Duality
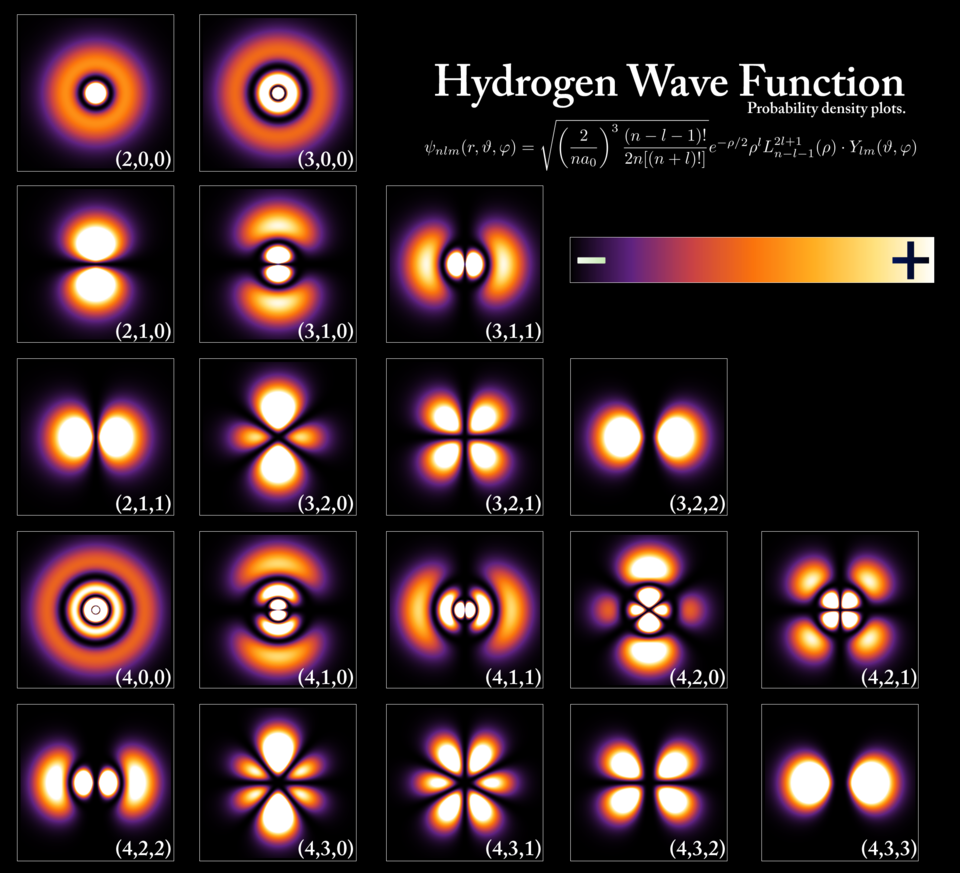
One of the most mind-bending aspects of quantum mechanics is the concept of wave-particle duality. This principle suggests that particles like electrons and photons can exhibit both wave-like and particle-like behavior, depending on how they are observed. For instance, electrons can act as particles when they collide with other objects, but they also behave like waves when they pass through slits or interfere with one another. This wave-particle duality was famously demonstrated in the double-slit experiment, where particles such as electrons or photons can create an interference pattern, typical of waves, when they are not being observed. But, when a measurement is made, the wave-like behavior collapses, and the particle behaves like a discrete entity.
This phenomenon forces us to rethink our traditional ideas about the nature of reality. Are particles truly both waves and particles simultaneously? Or is the act of observation itself altering the nature of the particles?
2. The Uncertainty Principle
Werner Heisenberg’s uncertainty principle, formulated in 1927, is another cornerstone of quantum mechanics. This principle asserts that certain pairs of properties, such as a particle’s position and momentum, cannot both be precisely measured at the same time. In other words, the more accurately we measure one of these properties, the less accurately we can measure the other.
The uncertainty principle has profound implications for our understanding of reality. It suggests that there is a fundamental limit to how much we can know about a quantum system, no matter how sophisticated our instruments are. This is a departure from classical physics, where measurements could, in theory, be infinitely precise.
3. Superposition
Another fascinating aspect of quantum mechanics is superposition. Superposition refers to the idea that a quantum system can exist in multiple states at once, and only when it is observed does it “collapse” into a single state. This concept is illustrated by Schrödinger’s famous thought experiment, where a cat in a box is both alive and dead at the same time, until the box is opened and the cat is observed.
Superposition is not just a theoretical idea; it has real-world consequences. For example, in quantum computing, superposition allows quantum bits, or qubits, to exist in multiple states simultaneously, enabling quantum computers to perform certain calculations much faster than classical computers. In fact, superposition is one of the key reasons why quantum mechanics has the potential to revolutionize computing, cryptography, and many other fields.
4. Entanglement
Quantum entanglement is perhaps the most mysterious and mind-boggling concept in quantum mechanics. It occurs when two or more particles become linked, such that the state of one particle is directly connected to the state of the other, no matter how far apart they are. When one particle is measured, the other particle’s state is instantly known, even if the particles are light-years apart.
This phenomenon was famously called “spooky action at a distance” by Albert Einstein, who initially rejected it as a flaw in quantum theory. However, entanglement has been repeatedly demonstrated in experiments, and it is now considered a real and essential part of the quantum world.
Entanglement has practical applications as well. It is a key ingredient in the emerging field of quantum computing and quantum cryptography, where it can be used to create ultra-secure communication systems.
5. The Observer Effect
The observer effect is another fascinating aspect of quantum mechanics. It refers to the idea that the act of observing or measuring a quantum system can influence its behavior. This idea challenges our classical intuitions about the separation between the observer and the observed. In the quantum world, the very act of observation seems to have an effect on the system being observed.
This idea was famously highlighted in the double-slit experiment, where particles behave differently when they are observed than when they are not. In some interpretations of quantum mechanics, this effect is so pronounced that it suggests that the observer plays an active role in creating reality.
6. Quantum Tunneling
Quantum tunneling is another phenomenon that defies classical intuition. It occurs when a particle passes through a barrier that it should not be able to pass through according to classical physics. In the quantum world, particles do not follow predictable paths; instead, there is a probability that they will “tunnel” through barriers that, classically, would be impenetrable.
This process is not just a theoretical curiosity; it has real-world applications. Quantum tunneling is responsible for the process of nuclear fusion in stars, including our sun. It also plays a role in technologies such as tunnel diodes and scanning tunneling microscopes.
The Copenhagen Interpretation and Other Interpretations
One of the major challenges of quantum mechanics is that it is not just mathematically difficult; it is also conceptually strange. In fact, one of the enduring debates in the field of quantum mechanics revolves around its interpretation—what the theory actually means.
The Copenhagen interpretation, formulated by Niels Bohr and Werner Heisenberg in the 1920s, is one of the most widely known interpretations. It suggests that a quantum system does not have definite properties until it is observed. In this view, the wave function—the mathematical description of a quantum system—describes the probabilities of different outcomes, not the outcomes themselves. The act of observation “collapses” the wave function, resulting in a definite outcome.
However, the Copenhagen interpretation is not without its critics. Some scientists have proposed alternative interpretations, such as the Many-Worlds Interpretation, which suggests that all possible outcomes of a quantum measurement occur in parallel universes. Others, like the de Broglie-Bohm interpretation, offer a deterministic view of quantum mechanics, in contrast to the probabilistic nature of the Copenhagen interpretation.
Quantum Mechanics in the Real World
While the ideas behind quantum mechanics may seem strange and abstract, their effects are felt all around us. The technologies that have been developed based on quantum mechanics are integral to our daily lives. For example:
- Semiconductors and Transistors: The modern electronics industry relies on quantum mechanics to design semiconductors and transistors, which are the building blocks of everything from smartphones to computers.
- Lasers: The principles of quantum mechanics are used in lasers, which are crucial to a wide range of technologies, from barcode scanners to medical treatments.
- Quantum Computing: Quantum computers, which exploit the principles of superposition and entanglement, are poised to revolutionize fields like cryptography, artificial intelligence, and materials science.
- Quantum Cryptography: The phenomena of quantum mechanics, such as entanglement, are being used to create ultra-secure communication systems, offering the potential for virtually unbreakable encryption.
The Future of Quantum Mechanics
Quantum mechanics is far from being a completed theory. Despite its success in explaining the behavior of matter on the microscopic scale, it still does not fully integrate with general relativity, the theory of gravity, which governs the macroscopic world. Physicists are actively working on theories that seek to unite these two pillars of modern physics, such as quantum gravity and string theory.
As we continue to explore the quantum realm, we are likely to uncover even more astonishing phenomena. Quantum mechanics challenges our deepest intuitions about reality and opens up possibilities that, just a century ago, would have seemed like science fiction.
In the end, quantum mechanics is not just a theory; it is a window into the very nature of the universe itself. The more we understand it, the more we realize how much there is yet to discover. Whether we are exploring the strange world of subatomic particles or harnessing quantum effects for new technologies, quantum mechanics is set to play a pivotal role in shaping the future of science and technology.
Quantum mechanics is not only the science of the tiny but also a profound challenge to the very way we understand the universe. It is an invitation to look beyond the familiar and embrace the weirdness that lies at the heart of nature.