Carbon capture, the process of isolating and removing carbon dioxide (CO2) from the atmosphere during industrial activities, has emerged as a critical tool in the fight against climate change. Industries such as cement production, steel manufacturing, and power generation are significant contributors to global greenhouse gas emissions. By capturing and preventing CO2 from being released into the atmosphere, carbon capture technologies can play a vital role in reducing emissions and mitigating global warming. However, despite its potential, existing carbon capture solutions face practical challenges, particularly concerning efficiency and environmental safety.
One of the most widely used technologies, amine scrubbing, involves chemical solvents that absorb CO2 from industrial emissions. While effective, this method presents several drawbacks. It is energy-intensive, requires high operating temperatures, and relies on potentially corrosive chemicals. These challenges have made it difficult to scale and deploy amine scrubbing on a global scale. To address these limitations, researchers have been exploring alternative approaches that are both efficient and environmentally friendly.
A promising breakthrough has come from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), where scientists are developing a carbon capture system using molecules called quinones. These organic compounds, dissolved in water, offer a safer, gentler, and potentially more efficient means of capturing CO2. A recent study published in Nature Chemical Engineering provides critical insights into how these aqueous quinone-based systems function, shedding light on their underlying mechanisms and paving the way for future advancements.
The research, led by Kiana Amini—now an assistant professor at the University of British Columbia—details the unique chemistry of quinone-mediated carbon capture systems. The study represents a collaborative effort, with Michael J. Aziz, a senior researcher at SEAS and an expert in materials and energy technologies, serving as the senior author. Aziz’s previous work in redox flow battery technology, which also utilized quinone chemistry, laid the foundation for these innovative carbon capture systems.
Quinones are small, organic molecules that occur naturally in crude oil and rhubarb. Their structure allows them to interact with CO2 in two distinct ways, enabling efficient capture, conversion, and release of the greenhouse gas. This ability to cycle CO2 capture and release multiple times makes quinones a highly attractive option for carbon capture technology. While researchers have long understood the basic principles of how quinones capture CO2, the exact contributions of their two capture mechanisms were previously unclear—until now.
In their study, the Harvard researchers delved into the detailed mechanisms of quinone-based carbon capture systems. Quinones capture CO2 through two processes: direct capture and indirect capture. These mechanisms operate simultaneously within the aqueous electrochemical system, making it challenging to discern their individual contributions without advanced analytical methods.
In the direct capture mechanism, quinones receive an electrical charge through a reduction reaction. This process gives the molecules a higher affinity for CO2, allowing them to bind with the gas and form stable chemical complexes known as quinone-CO2 adducts. Essentially, the quinone molecules act as chemical “magnets,” attaching themselves to CO2 molecules and removing them from the surrounding environment.
The indirect capture mechanism, on the other hand, involves a shift in the chemical properties of the solution. When quinones are charged, they consume protons from the surrounding solution, which raises the solution’s pH level. The increased alkalinity creates an environment where CO2 reacts with water to form bicarbonate or carbonate ions. This secondary pathway provides an additional method for removing CO2 from the system.
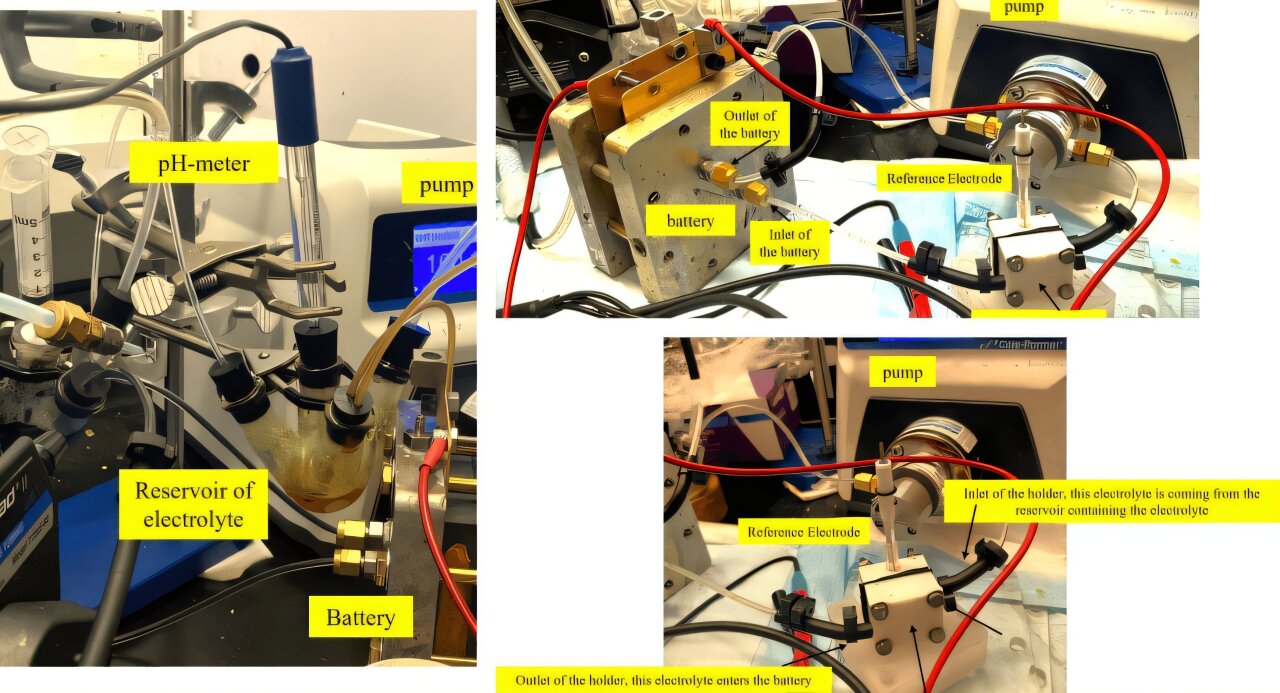
To quantify the contributions of these two mechanisms, the research team developed innovative real-time experimental techniques. The first method involved the use of reference electrodes to measure voltage differences between quinones and the quinone-CO2 adducts. These voltage signatures provided insights into the direct capture process. The second method employed fluorescence microscopy to distinguish between different chemical states—oxidized quinones, reduced quinones, and quinone-CO2 adducts. By analyzing the unique fluorescence patterns of these compounds, researchers were able to monitor their concentrations with remarkable precision and in near real-time.
These advanced techniques allowed the team to “open the black box” of quinone-mediated carbon capture, revealing how each mechanism contributes to the system’s overall performance. By quantifying the roles of direct and indirect capture, the researchers gained critical insights that will guide the future design of optimized systems tailored to specific industrial applications.
The potential implications of this research are significant. Aqueous quinone-based carbon capture systems have several advantages over traditional methods. They use water as a solvent, avoiding the corrosive chemicals associated with amine-based systems. Additionally, quinones are abundant, inexpensive, and can be derived from natural sources, making them a sustainable choice for large-scale implementation.
Despite these advantages, challenges remain. One notable obstacle is the system’s sensitivity to oxygen, which can degrade performance by interfering with the capture process. Addressing these limitations will require further research and refinement. However, the findings from this study provide a solid foundation for overcoming such hurdles. By understanding the precise chemistry of quinone-mediated carbon capture, researchers can develop more robust and efficient systems capable of addressing the diverse needs of different industries.
The research also highlights the versatility of quinone chemistry beyond carbon capture. Michael J. Aziz’s earlier work on redox flow batteries demonstrated how quinones could be used to store energy for grid applications. This dual applicability underscores the potential for quinones to drive innovation in multiple fields of energy and environmental science. By leveraging this unique chemistry, scientists can develop technologies that not only capture carbon but also contribute to the broader transition to sustainable energy systems.
Reference: Kiana Amini et al, In situ techniques for aqueous quinone-mediated electrochemical carbon capture and release, Nature Chemical Engineering (2024). DOI: 10.1038/s44286-024-00153-y