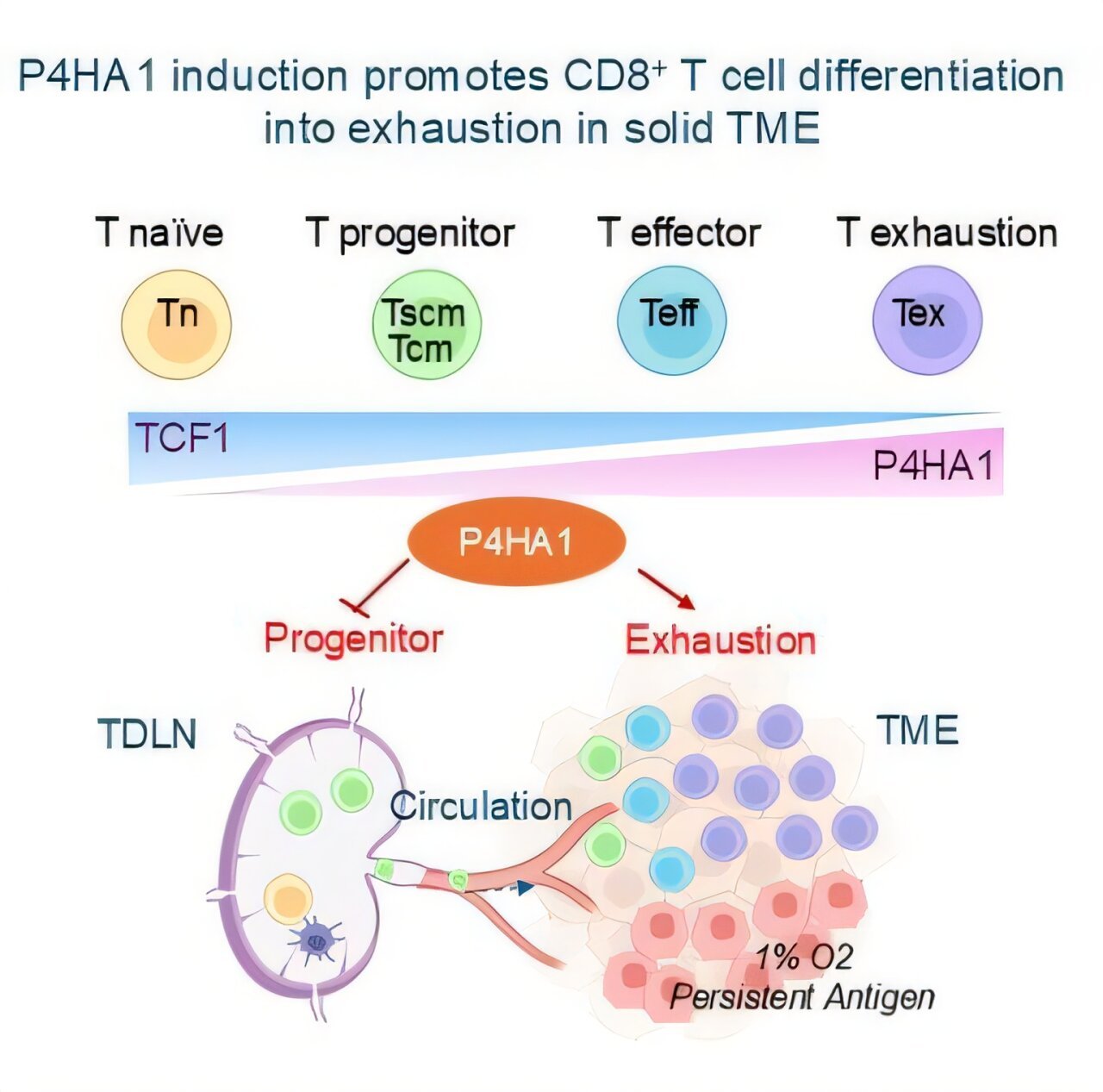
Researchers at ASTAR’s Genome Institute of Singapore (ASTAR GIS) have uncovered a key discovery in the ongoing battle against cancer, revealing how a specific enzyme, P4HA1 prolyl hydroxylase, plays a crucial role in the immune system’s ability to fight solid cancers. The research, published in Cancer Cell in December 2024, has the potential to revolutionize current cancer treatments by targeting a metabolic enzyme that undermines immune response, offering hope for both new therapeutic strategies and a better way to track the effectiveness of immunotherapies.
The Role of P4HA1 in T Cells and Cancer Immunity
CD8+ T cells, a type of white blood cell, are one of the body’s most critical defenses against cancer. They are the main immune cells responsible for identifying and destroying cancerous cells. However, in many cancers, especially solid tumors, the function of these vital cells is often severely weakened. Scientists have long sought the underlying causes of this immune dysfunction, and the research team at A*STAR GIS discovered a surprising culprit: the enzyme P4HA1.
P4HA1, a prolyl hydroxylase enzyme, typically contributes to collagen production, a vital component of the extracellular matrix. However, it is now evident that its role extends to regulating the metabolism and function of immune cells, particularly T cells. In the tumor microenvironment (TME), P4HA1 is upregulated in exhausted CD8+ T cells, significantly impairing their ability to generate energy and function properly. This disruption of energy production leads to weakened immune responses and an inability of T cells to form a lasting anti-cancer memory, an essential aspect for long-term immunity and protection against tumor relapse.
The A*STAR GIS study found that the expression of P4HA1 is significantly elevated in T cells within solid tumors, leading to energy dysregulation, immune exhaustion, and poor T cell function. This impairment is especially problematic because it prevents immune cells from adapting and effectively combating cancer in the long term. Furthermore, P4HA1 levels in blood immune cells were found to increase as cancer progresses and relapse occurs. These findings suggest that P4HA1 not only weakens the immune system’s defenses but can also serve as a potential biomarker for tracking cancer progression, immunotherapy responses, and relapse.
The Potential of Targeting P4HA1 for Tumor Treatment
The breakthrough from A*STAR GIS goes beyond merely understanding the role of P4HA1. The researchers were able to show that inhibiting P4HA1 through the use of small molecule compounds helped restore proper immune cell function. This inhibition allowed the immune system, particularly the CD8+ T cells, to recover their full potential in recognizing and attacking cancer cells, ultimately shrinking tumors and providing durable immunity against cancer relapse. This approach was effective in mouse models, including those with immune-resistant tumors, demonstrating that P4HA1 inhibition could be an essential strategy for overcoming some of the toughest cancer cases.
One of the most promising implications of these findings is the potential for combining P4HA1 inhibition with existing cancer treatments. In particular, P4HA1 inhibition could be used to enhance the effectiveness of CAR-T (Chimeric Antigen Receptor T-cell) therapy. CAR-T is a powerful treatment that involves genetically modifying a patient’s T cells to more effectively target cancer cells. However, its effectiveness has often been hampered by exhaustion and low activity of the T cells in the hostile tumor environment. Targeting P4HA1 in the tumor microenvironment could improve CAR-T cell strength and effectiveness, leading to better treatment outcomes and potentially enabling it to tackle cancers that have previously been resistant to such therapies.
P4HA1 as a Biomarker for Cancer Monitoring
Another significant revelation from this research is that P4HA1 levels in immune cells correlate with patients’ responses to immunotherapy. By analyzing immune cells in tumor-draining lymph nodes (TDLNs) and peripheral blood, the scientists discovered that elevated P4HA1 levels were linked to poor immune responses, cancer relapse, and resistance to treatments such as immune checkpoint inhibitors. Given this, P4HA1 has the potential to serve as both a target for therapeutic intervention and a biomarker for monitoring the progression of cancer and patient responses to immunotherapies.
Notably, because P4HA1 is expressed at low levels in naïve T cells and memory T cells under normal conditions but becomes significantly upregulated in activated T cells, particularly exhausted ones in the tumor microenvironment, it is highly specific to cancer-related immune dysfunction. This specificity allows for a more tailored approach to cancer treatment, as inhibiting P4HA1 selectively targets these problematic T cells without affecting the broader immune system.
Advancing Cancer Immunotherapies and Personalized Medicine
The discoveries made by the ASTAR GIS team could have wide-ranging implications for the future of cancer treatment. As Prof. Yu Qiang, Senior Group Leader at ASTAR GIS, pointed out, the work bridges fundamental research with clinical applications. The next steps will involve developing optimized P4HA1-targeting strategies, including chemical inhibitors and advanced CAR-T cell platforms. These developments could lead to more efficient and feasible therapies for solid tumors, which are often more difficult to treat than hematological cancers.
Furthermore, Dr. Wan Yue, Executive Director at A*STAR GIS, emphasized the potential of using P4HA1 as a biomarker to monitor cancer progression and assess the effectiveness of immunotherapies. This ability to personalize treatment could greatly improve outcomes for cancer patients, enabling more precise monitoring, adapting therapies to individual needs, and improving overall patient safety and care.
This research contributes to a broader understanding of how the immune system interacts with cancer and opens up new possibilities for combating immune resistance in tumors. By focusing on systemic immunity, the study highlights the importance of not only directly targeting the tumor but also sustaining an immune response over time. This systemic approach is crucial for achieving long-term control of cancer and reducing the likelihood of relapse.
Conclusion
The discovery that P4HA1 is a key metabolic regulator that disrupts immune function within the tumor microenvironment represents a significant step forward in cancer research. This work sheds light on why the immune system often fails to recognize and attack solid tumors and offers an innovative target for therapy. By inhibiting P4HA1, scientists were able to restore proper immune function, shrink tumors, and prevent relapse in mouse models. Moreover, the discovery of P4HA1’s role in immune cell dysfunction also offers the potential for improving current cancer immunotherapies and providing a new way to monitor cancer progression. Ultimately, this research paves the way for the development of more effective, personalized cancer treatments, giving hope for better outcomes for patients with solid tumors.
As scientists work to refine P4HA1-targeting therapies and integrate these findings into clinical practice, the fight against cancer becomes a bit more hopeful, with the promise of targeted treatments that could offer lasting solutions to some of the most challenging forms of the disease.
Reference: Shijun Ma et al, Targeting P4HA1 promotes CD8+ T cell progenitor expansion toward immune memory and systemic anti-tumor immunity, Cancer Cell (2024). DOI: 10.1016/j.ccell.2024.12.001