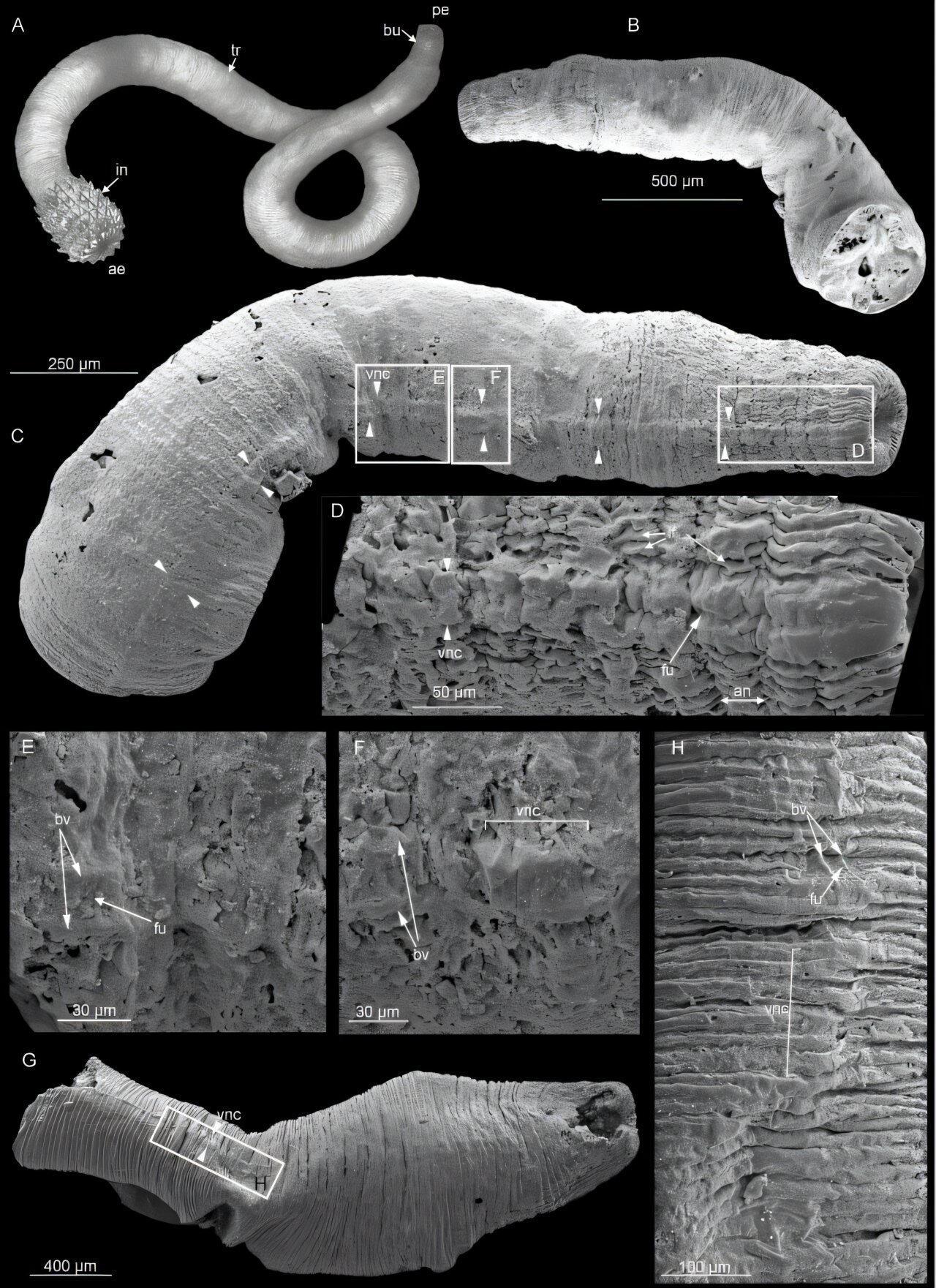
An international team of scientists has unveiled a groundbreaking discovery about the evolution of the ventral nerve cord, a vital component of the central nervous system in ecdysozoan animals, a group that includes organisms such as insects, nematodes, and priapulid worms. Their research, detailed in the paper “Preservation and early evolution of scalidophoran ventral nerve cord,” published in Science Advances, sheds light on the ancient origins of these nerve structures during the basal Cambrian period, over 500 million years ago.
This work was a collaborative effort by experts from institutions around the globe, including Dr. Deng Wang of Northwest University, Dr. Jean Vannier from Université de Lyon, Dr. Chema Martin-Durán at Queen Mary University of London, and Dr. María Herranz of Rey Juan Carlos University. They meticulously analyzed exceptionally well-preserved fossils from renowned Cambrian deposits, gaining unprecedented insight into the neural architecture of ancient animals that hold key positions in the evolutionary tree.
The study focused on early representatives of the Scalidophora, a subgroup of Ecdysozoa, which includes priapulids, kinorhynchs (“mud dragons”), and loriciferans. These small but pivotal groups represent critical links in understanding how nervous system structures, particularly the ventral nerve cord, developed over evolutionary time. Their findings not only reveal how the ventral nerve cord may have originated but also provide fresh perspectives on its variations among ecdysozoan species.
The ventral nerve cord serves as the main communication highway for transmitting signals between the brain and the body in ecdysozoan organisms. Priapulids, for instance, feature a single ventral nerve cord, while kinorhynchs possess paired nerve cords connected to paired ganglia. The loriciferans’ nervous system also closely resembles that of arthropods, such as insects and crabs, which have paired nerve cords. This raised fascinating questions: Did the ancestral ecdysozoan species originally have a single or a paired ventral nerve cord? Moreover, do the similarities between distantly related groups like loriciferans and arthropods stem from shared ancestry or convergent evolution?
The fossils studied include remarkable specimens from diverse Cambrian deposits. These include the Fortunian Kuanchuanpu Formation, showcasing species like Eopriapulites and Eokinorhynchus; the Chengjiang Biota, which preserves organisms such as Xiaoheiqingella and Mafangscolex; and the Wuliuan Ottoia prolifica. These ancient sites are among the world’s most significant Cambrian fossil repositories, offering scientists invaluable insights into early animal life. The fossils revealed elongate impressions running along the ventral sides of these creatures—structures that closely resemble the modern ventral nerve cords of priapulids.
According to Dr. Deng Wang and Dr. Jean Vannier, these fossilized impressions represent ancient single ventral nerve cords, a significant clue about the ancestral condition for scalidophorans. Through rigorous phylogenetic analyses, the research team concluded that the single ventral nerve cord likely represents the original state for scalidophorans. They further proposed that the common ancestor of all ecdysozoans possessed a similar simple nerve cord configuration.
Dr. Chema Martin-Durán added that the paired nerve cords seen in arthropods, loriciferans, and kinorhynchs most likely evolved independently, diverging from the ancestral single cord design. These paired nerve cords represent evolutionary adaptations rather than shared ancestral traits. This distinction highlights the extraordinary diversity of nervous system structures within the ecdysozoan lineage, driven by natural selection to meet specific ecological and functional challenges.
The study delved deeper into the connection between paired nerve cords and body segmentation, another hallmark of certain ecdysozoans. Loriciferans, kinorhynchs, and panarthropods—an ecdysozoan group including arthropods, tardigrades, and onychophorans—exhibit segmented body plans that correspond with modifications in their nervous systems. The development of paired nerve cords and ganglia likely co-evolved alongside segmentation, providing enhanced coordination for movement. This evolutionary adaptation is particularly advantageous for species with appendages, as it supports more complex and efficient locomotion.
Dr. María Herranz elaborated that these changes in nervous and muscular systems during the Precambrian-Cambrian transition likely coincided with the rise of appendages. These physical developments would have allowed ancient ecdysozoans to navigate their environments with unprecedented dexterity, opening new ecological opportunities. As a result, they could better exploit resources, evade predators, and adapt to shifting environmental conditions, setting the stage for the incredible diversity observed among modern ecdysozoans.
By tracing the evolutionary steps of the ventral nerve cord, this research offers broader insights into the early evolution of complex animal life. It also underscores the critical role that fossil evidence plays in piecing together the intricate puzzle of life’s history. The fossil record serves as a window into the past, revealing how ancient anatomical structures, including the nervous system, shaped the success and diversification of life on Earth.
Furthermore, the study reinforces the idea that ecdysozoan evolution was not a linear progression but a dynamic, branching process influenced by multiple evolutionary pressures. While some groups retained the ancestral single nerve cord, others independently acquired paired configurations, reflecting the remarkable adaptability of life. These changes underscore the interplay between nervous system structures and other traits, such as body segmentation and appendages, in driving evolutionary innovation.
In the larger context, the findings have implications beyond the field of paleobiology. They provide a valuable framework for comparative studies on modern ecdysozoans and other animals. Understanding how nervous systems evolve offers key insights into the relationships between anatomy, behavior, and ecology, helping scientists answer fundamental questions about life’s complexity.
This research also highlights the importance of interdisciplinary collaboration in addressing profound questions about evolution. By bringing together expertise in paleontology, comparative anatomy, and phylogenetics, the team bridged gaps between fossils and living organisms, offering a cohesive narrative about early nervous system evolution in ecdysozoans.
The work has opened doors for future research aimed at exploring similar evolutionary phenomena in other groups of organisms. Whether investigating the origins of multicellularity, the evolution of sensory systems, or the diversification of body plans, studies like this illuminate pathways of innovation that define the story of life.
Ultimately, this discovery represents a testament to the power of scientific inquiry to uncover hidden aspects of our planet’s deep history. As our understanding of ancient nervous systems grows, so too does our appreciation for the evolutionary processes that have shaped the rich diversity of life observed today. Through fossils, researchers continue to piece together the remarkable journey of evolution, advancing our knowledge of life’s past and deepening our curiosity about its future.
Reference: Deng Wang, Preservation and early evolution of scalidophoran ventral nerve cord, Science Advances (2025). DOI: 10.1126/sciadv.adr0896. www.science.org/doi/10.1126/sciadv.adr0896