Hunger and food intake are controlled by complex processes in the brain, which trigger a series of mechanisms that initiate eating when the body requires fuel. A recent study conducted by researchers from Baylor College of Medicine and the University of Texas Health Science Center at Houston, published in the journal Metabolism, delves into the brain circuits and chemical messengers involved in regulating meal initiation and food consumption. These findings offer insights into the brain’s intricate pathways of feeding behavior and have significant implications for understanding and treating obesity, a growing health crisis worldwide.
One of the key components in this study is serotonin, a neurotransmitter widely recognized for its role in suppressing appetite and food intake. Over the years, serotonin’s impact on regulating food consumption led to the development of serotonin-targeting drugs designed to combat obesity. However, as the study points out, while serotonin holds promise, many of these drugs led to adverse side effects and are no longer in common use. This highlights the critical need for a deeper understanding of the brain’s complex regulation of food intake, which can lead to more effective, safer therapies for obesity management.
Dr. Yong Xu, a professor of pediatrics and nutrition at Baylor College of Medicine, and corresponding author of the study, points out that although serotonin has long been known to play an inhibitory role in feeding, the exact mechanisms and brain circuits involved remain only partially understood. Previous research primarily focused on serotonin itself, but this study delves further by examining other brain circuits and neurotransmitters that influence serotonin-producing neurons and their function in regulating food intake. By doing so, Xu and his team hope to uncover novel approaches to better target the brain’s feeding pathways.
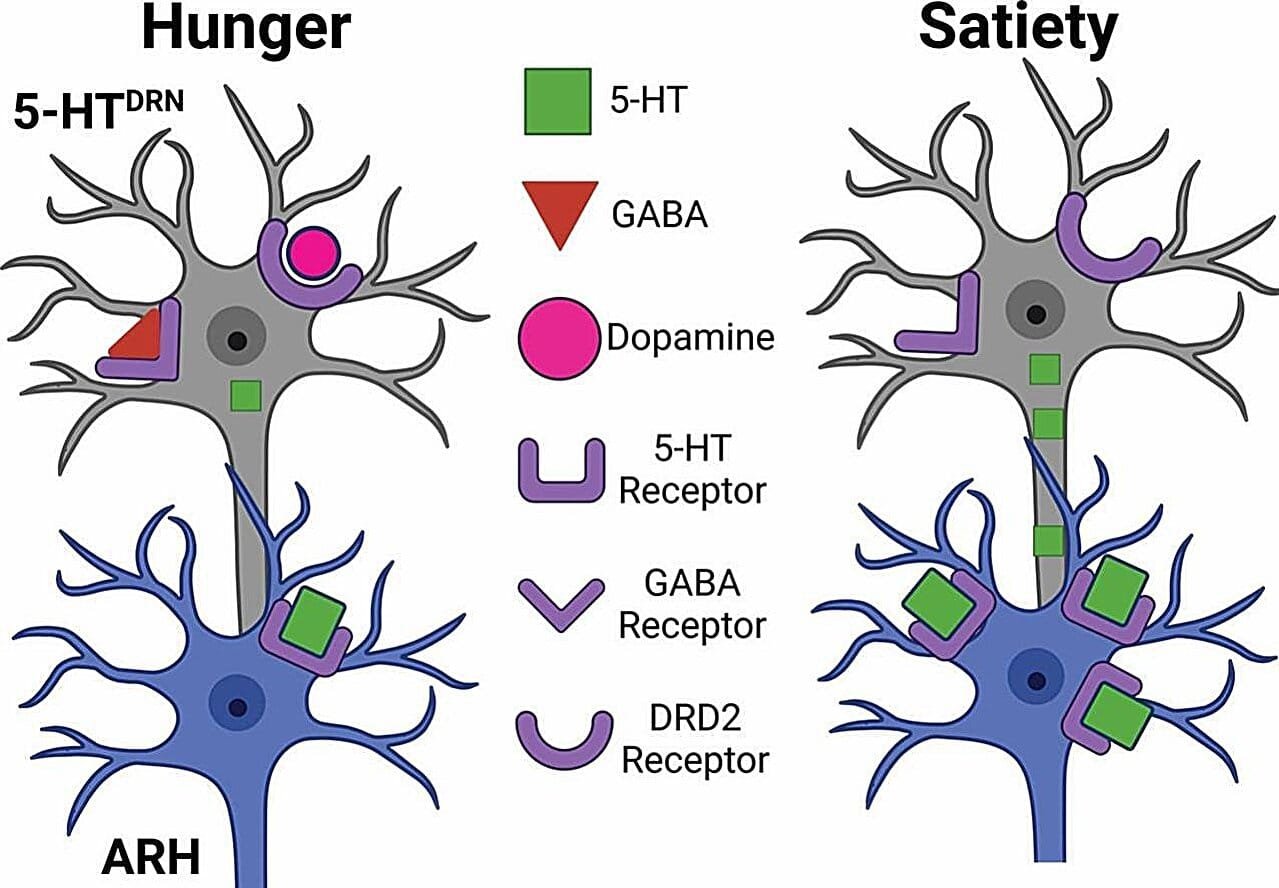
Serotonin is synthesized in the brain’s dorsal raphe nucleus (DRN), located in the midbrain. Neurons in the DRN release serotonin and send projections to several brain regions, including the arcuate nucleus of the hypothalamus (ARH), a key site involved in regulating hunger. The team’s findings reveal that in states of hunger, two other neurotransmitters—gamma-aminobutyric acid (GABA) and dopamine—play essential roles in controlling the serotonin-producing neurons. They found that GABA and dopamine act to inhibit these neurons during hunger, thereby reducing serotonin’s suppressive effects and allowing the brain to initiate the process of meal consumption. As the animals consume food and achieve satiety, these inhibitory signals subside, resulting in an increase in serotonin production, which in turn serves to inhibit further eating by sending signals back to the ARH.
The researchers also noted the unique synergy between GABA and dopamine in inhibiting serotonin. When both neurotransmitters are active at the same time, they appear to exert a more significant inhibitory effect on serotonin-producing neurons than if each was acting alone. This discovery sheds light on the precise neurochemical balance required for regulating food intake, with GABA and dopamine serving as crucial signals that help to control feeding during periods of hunger.
The implications of this study extend far beyond theoretical interest. Understanding how serotonin is regulated by GABA and dopamine is a vital piece of the puzzle in uncovering the brain’s mechanisms for food intake and weight control. By understanding the dynamics of neurotransmitter interaction and their influence on meal initiation, researchers can begin to envision new and more targeted approaches to developing obesity drugs. Unlike older serotonin-based treatments, which were fraught with side effects and limited efficacy, a better understanding of these regulatory circuits might lead to the design of drugs that more precisely control eating behaviors with fewer negative consequences.
The discovery opens the door to future research exploring how other stages of feeding are regulated by brain circuits and neurotransmitters. While this study primarily focused on the early stages of meal initiation—when an animal begins eating due to hunger—there are other critical phases of feeding behavior, including satiety and overconsumption. Exploring how the brain signals satiety and prevents overeating could lead to even more comprehensive treatments for obesity. Moreover, targeting neurotransmitter systems such as GABA and dopamine, in addition to serotonin, could enable more precise regulation of feeding behaviors, helping individuals achieve healthier eating patterns and more sustainable weight management.
Dr. Xu’s research aims not only to understand the immediate processes that control food intake but also to elucidate how the brain manages overall body weight. A sophisticated and multi-faceted understanding of how neurotransmitters and brain circuits work together to regulate feeding can contribute to improving therapeutic strategies for conditions like obesity and related metabolic disorders. With nearly 40% of adults in the United States considered obese, addressing the underlying mechanisms of hunger, meal initiation, and food consumption is a priority for public health. Drugs that effectively manage obesity must avoid or minimize side effects while promoting healthy eating behaviors, and the more knowledge researchers acquire about the underlying brain circuits involved in food intake, the more refined the therapies can become.
The findings from this research contribute significantly to the broader field of neurobiology and nutrition. The interactions between different neurotransmitters involved in controlling eating—such as serotonin, GABA, and dopamine—are essential for maintaining homeostasis in the body and regulating eating patterns. This knowledge is crucial for developing drugs that regulate these pathways with a focus on meal initiation, appetite suppression, and energy balance without risking undesirable side effects.
Additionally, understanding how neurotransmitters like serotonin interact within specific brain circuits could pave the way for other innovations in medicine. For example, therapies that could enhance the brain’s ability to regulate feeding in response to internal hunger signals may be beneficial in managing conditions not only limited to obesity, such as anorexia nervosa, or conditions where appetite is compromised.
Looking ahead, Dr. Xu and his colleagues are interested in expanding their research to explore how other signals and circuits influence the later stages of feeding behavior. While the role of GABA and dopamine in meal initiation is now clearer, there remains much to be learned about the brain mechanisms involved in the transition from hunger to fullness. By studying the transitions between different feeding stages, the researchers hope to uncover new avenues for preventing overeating and promoting balanced, healthy food intake.
Reference: Kristine M. Conde et al, Serotonin neurons integrate GABA and dopamine inputs to regulate meal initiation, Metabolism (2024). DOI: 10.1016/j.metabol.2024.156099