Research into neuropsychiatric disorders has taken an innovative turn with the discovery that specific risk genes associated with schizophrenia might be expressed in retinal cells. The retina, which is a direct outgrowth of the brain, has long been a subject of interest for neuroscientists because of its genetic similarities to the brain. Now, researchers have successfully combined different datasets to uncover critical connections between these retinal cells and a range of neuropsychiatric disorders. By investigating the genetic risk factors for schizophrenia and other conditions, they found compelling evidence that retinal cell dysfunction might reflect broader biological issues affecting communication within the brain.
The findings, published in JAMA Psychiatry, signal a potential breakthrough in how we understand the underlying mechanisms of schizophrenia and other mental health conditions. What makes these insights especially exciting is the possibility of using the retina as a non-invasive proxy to study changes occurring in the brain.
Retinal Alterations and Schizophrenia
In a study spearheaded by Florian Raabe and Emanuel Boudriot from the Max Planck Institute for Psychiatry and Ludwig Maximilian University (LMU Munich), the research team focused on the genetic intersections between neuropsychiatric disorders and retinal cell biology. A previous investigation by the Max Planck Translational Deep Phenotyping group had already identified retinal changes in patients with schizophrenia, alterations that were especially pronounced in individuals at higher genetic risk for the disorder. These retinal changes were previously assumed to be associated with common comorbidities, such as obesity or diabetes. However, the new findings suggest that these alterations may stem from processes directly linked to schizophrenia itself, potentially offering clues to the core pathophysiological mechanisms of the disorder.
Schizophrenia, a complex psychiatric illness characterized by delusions, hallucinations, and cognitive deficits, has long been known to affect the brain’s ability to process and integrate information from the outside world. Scientists have traditionally struggled to pinpoint specific biological markers of schizophrenia, but recent advances in genetics have shown that certain risk genes are more strongly associated with particular neural structures and functions. The retina, with its close connection to the brain’s neural circuits, may be an ideal tissue for examining these disruptions, making it easier to explore the biology of psychiatric disorders in a more accessible and cost-effective way.
In their latest study, Raabe and Boudriot used genetic risk data from several major neuropsychiatric disorders—schizophrenia, bipolar disorder, Alzheimer’s disease, and multiple sclerosis (MS), to name a few—and compared this information with detailed RNA sequencing data from retinal cells. This large-scale, multi-dataset approach allowed the researchers to pinpoint which risk genes are linked to specific types of retinal cells in these conditions.
Synaptic Dysfunction and Schizophrenia: New Insights from the Retina
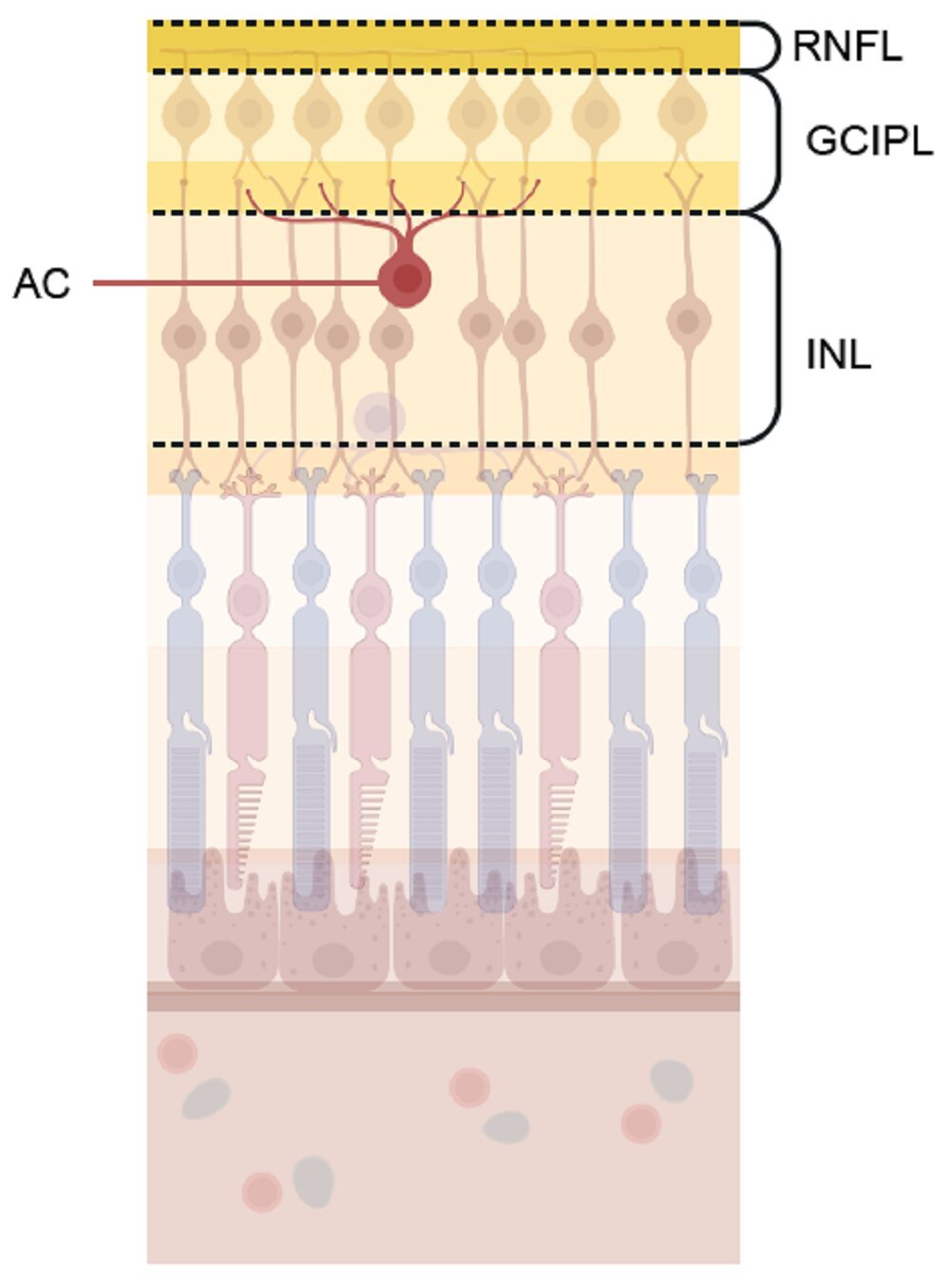
The study identified key genetic connections in schizophrenia that had not been clear in prior research. In particular, risk genes for schizophrenia appeared to affect amacrine cells, a specific class of retinal neurons. Amacrine cells are crucial for synaptic function and are integral to the way retinal neurons communicate with each other. They play a role in transmitting and modulating signals between the photoreceptor cells in the retina and other types of neurons, shaping the signals sent to the brain. Synaptic communication in these cells is vital for maintaining the integrity of visual processing and neural coordination.
The researchers suspect that the impairment of these amacrine cells may reflect similar synaptic problems in the brain. Just as amacrine cells in the retina rely on effective synaptic function for communication, neurons in the brain do the same. The shared mechanisms between retinal cells and brain neurons offer tantalizing clues about the neural disruptions that might be present in individuals with schizophrenia, helping us better understand why symptoms like cognitive impairments and altered perception arise in those affected by the disease.
Moreover, the study found that the higher the genetic risk for schizophrenia, the thinner the synaptic layer was in the amacrine cells in the retina. This structural observation was confirmed by an extensive dataset derived from over 36,000 healthy participants in a UK-based biobank study, lending further weight to the idea that schizophrenia-linked genetic risk affects synaptic structure in both the retina and the brain.
Implications for Understanding Other Neuropsychiatric Disorders
The implications of this research are not confined to schizophrenia alone. Through their investigation, Raabe and Boudriot also observed that retinal cell alterations could be associated with other neuropsychiatric and neurodegenerative conditions. For example, the study found a strong connection between genetic risk for multiple sclerosis (MS) and retinal immune cells. MS, a disease of the central nervous system characterized by autoimmune responses that target the myelin sheath of nerve cells, appears to have identifiable genetic markers present in the immune cells of the retina.
Other disorders, such as bipolar disorder, depression, Parkinson’s disease, Alzheimer’s disease, and even stroke, have also been linked to alterations in the retina. This new genetic research suggests that the retina might serve as a window into the pathophysiology of a range of neuropsychiatric and neurodegenerative diseases, offering opportunities for earlier detection and improved understanding of these conditions.
A New Avenue for Precision Medicine
One of the most exciting prospects stemming from this research is the potential to use the retina as a diagnostic tool for neuropsychiatric diseases. As Raabe explains, “Finding this impairment in the eye suggests that processes in the retina and in the brain are very similar—this would make the retina a great proxy for studying neuronal disorders, because we can examine the retina of patients with a much higher resolution than the brain.”
By understanding how genetic risk factors alter retinal cells, scientists can potentially identify biomarkers that predict disease before symptoms become apparent. Additionally, by identifying the specific cells and structures involved in disease processes, researchers can work toward more precise and individualized treatment options. The ability to target these mechanisms in the retina could open up new therapeutic possibilities, particularly for conditions like schizophrenia, where current treatment options are often generalized and not personalized to the underlying biological pathways of each patient.
This study’s combination of genetic risk data, advanced sequencing techniques, and structural analysis positions the retina as a highly valuable tool for unraveling the complexities of the brain and mental health disorders. It heralds a future in which retinal imaging might play an instrumental role in clinical settings, providing a non-invasive method to study neuronal dysfunction across a variety of conditions.
Conclusion: The Retinal Frontier in Neuropsychiatric Research
In conclusion, the research conducted by Raabe, Boudriot, and their colleagues at the Max Planck Institute of Psychiatry reveals groundbreaking insights into the genetic connection between retinal cell biology and neuropsychiatric disorders. By analyzing large datasets, the researchers were able to uncover important associations between genetic risk factors for schizophrenia, amacrine cells, and the overall synaptic connectivity in the retina. These findings suggest that impaired synaptic biology may not only affect neurons in the retina but also play a crucial role in schizophrenia’s onset and progression.
The implications of this study extend far beyond the retina, however. They offer potential pathways for better understanding and diagnosing a variety of mental health disorders, from schizophrenia to MS. By using the retina as a model for understanding the brain, researchers are opening up new ways of thinking about how to study and ultimately treat neuropsychiatric diseases. The hope is that, with this deeper understanding, more targeted and effective treatments can be developed, advancing the field of precision medicine and benefiting countless patients worldwide.
Reference: Emanuel Boudriot et al, Genetic Analysis of Retinal Cell Types in Neuropsychiatric Disorders, JAMA Psychiatry (2025). DOI: 10.1001/jamapsychiatry.2024.4230
Behind every word on this website is a team pouring heart and soul into bringing you real, unbiased science—without the backing of big corporations, without financial support.
When you share, you’re doing more than spreading knowledge.
You’re standing for truth in a world full of noise. You’re empowering discovery. You’re lifting up independent voices that refuse to be silenced.
If this story touched you, don’t keep it to yourself.
Share it. Because the truth matters. Because progress matters. Because together, we can make a difference.
Your share is more than just a click—it’s a way to help us keep going.