Researchers at University College London (UCL) and the UK Dementia Research Institute (UK DRI) have uncovered significant genetic differences that influence both lifespan and the risk of developing Alzheimer’s disease. This groundbreaking study, published in Brain, identifies genetic variations in specific brain cells—particularly microglia, which are immune cells in the brain, and oligodendrocytes, the cells responsible for supporting nerve cells. These genetic differences, the study suggests, play a crucial role in both the aging process and the development of Alzheimer’s disease.
The research is part of a broader effort to understand the biological underpinnings of aging and Alzheimer’s, with the hope of identifying potential new therapeutic targets for drug discovery. According to Dr. Dervis Salih, the senior author of the study and a researcher at the UCL Queen Square Institute of Neurology, as well as the UK Dementia Research Institute, these findings may pave the way for innovative treatments that could slow brain aging and mitigate the progression of Alzheimer’s. Dr. Salih emphasized the importance of understanding how certain brain cells change with age, offering valuable insights into the aging brain and the possibility of developing preventative therapies for Alzheimer’s disease.
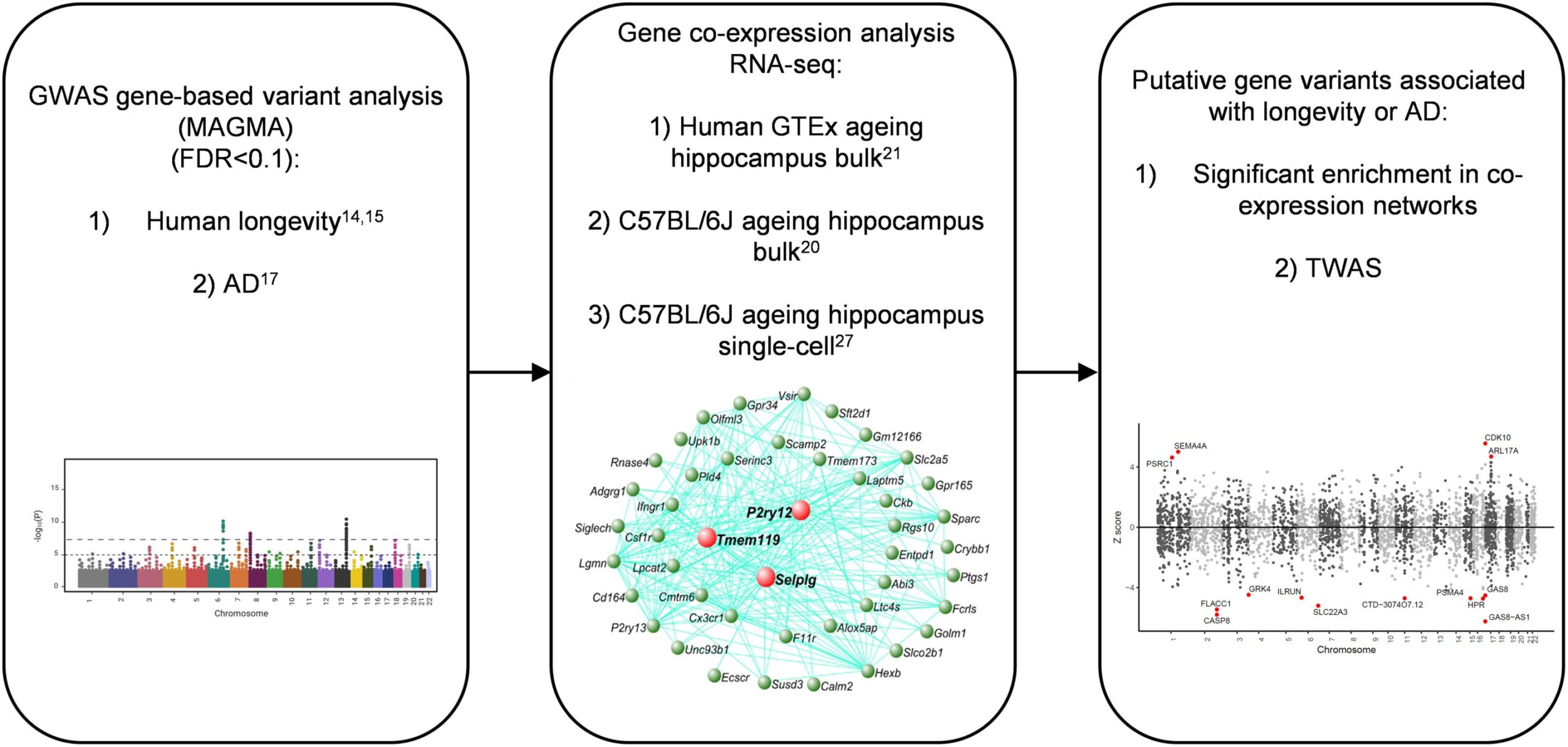
The researchers used large-scale datasets to explore the genetic factors influencing both aging and Alzheimer’s disease. These datasets included genetic data from over 21,000 people with Alzheimer’s and nearly 42,000 people without the disease, sourced from the International Genomics of Alzheimer’s Projects (IGAP). Additionally, they utilized European ancestry data, encompassing over 300,000 individuals for aging-related traits such as the length of time a person remains healthy, over 11,000 people for data on longevity, and more than 1 million parents’ lifespan data from a study at the University of Edinburgh. The combination of these datasets allowed the researchers to examine the relationship between genetic variants and both aging and Alzheimer’s risk in a detailed and comprehensive way.
To gain deeper insights into gene activity and how it changes with aging and Alzheimer’s, the team also utilized RNA sequencing data. RNA sequencing helps researchers understand which genes are active in cells and how their activity changes in response to aging and disease. This technique was applied to both human and mouse models to analyze gene expression in brain cells, further shedding light on the molecular processes involved in aging and Alzheimer’s.
The study’s findings revealed that microglia and oligodendrocytes undergo significant changes as individuals age, and these changes are linked to an increased risk of developing Alzheimer’s disease. While these cellular changes were observed in both mice and humans, the genetic connections to Alzheimer’s were only found in humans, suggesting that the aging process in human brain cells might make them more vulnerable to the disease. This indicates that certain genetic variants may predispose individuals to dementia, depending on how their brain cells respond to the aging process.
One of the most important discoveries in the study was the identification of specific genetic variants that were associated with both aging and Alzheimer’s. A notable example is the APOE gene, which has long been associated with Alzheimer’s risk. The APOE gene provides instructions for creating a protein called apolipoprotein E, which helps transport fats and cholesterol in the bloodstream. The study found that certain variants of APOE had a strong influence on both aging and Alzheimer’s disease risk. This highlights a sequential process in which aging is followed by dementia, suggesting that genetic variations related to aging may set the stage for the development of Alzheimer’s later in life.
The findings offer a potential explanation for why some individuals may develop Alzheimer’s in their 70s or 80s, while others remain mentally sharp well into their later years. The genetic factors identified in the study could explain why some people are genetically predisposed to age in a way that primes their brains for dementia, while others remain less affected by the aging process.
Dr. Salih further explained that the genetic differences in microglia and oligodendrocytes influence how these cells function as individuals age, either in a healthy or disease-related manner. Microglia play a central role in the immune response of the brain, and their activation can contribute to inflammation, a hallmark of Alzheimer’s disease. Oligodendrocytes, on the other hand, are responsible for maintaining the integrity of nerve cells by providing them with support and insulation. As these cells undergo age-related changes, they can become dysfunctional, potentially contributing to the onset and progression of Alzheimer’s.
By gaining a better understanding of how these brain cells change with age, the researchers believe it may be possible to develop new tests and biomarkers that could help slow the aging of the brain and delay the progression of Alzheimer’s disease. These biomarkers could be used to detect early signs of Alzheimer’s, potentially leading to earlier interventions that could improve the quality of life for those at risk.
The implications of this study extend beyond just Alzheimer’s research. By investigating how aging-related genetic changes affect brain cells, the findings offer insights into broader questions of aging and longevity. As the global population continues to age, understanding the genetic and cellular mechanisms that contribute to age-related diseases like Alzheimer’s could be crucial for developing strategies to promote healthier aging and improve the lives of older individuals.
Reference: Andrew C Graham et al, Human longevity and Alzheimer’s disease variants act via microglia and oligodendrocyte gene networks, Brain (2025). DOI: 10.1093/brain/awae339