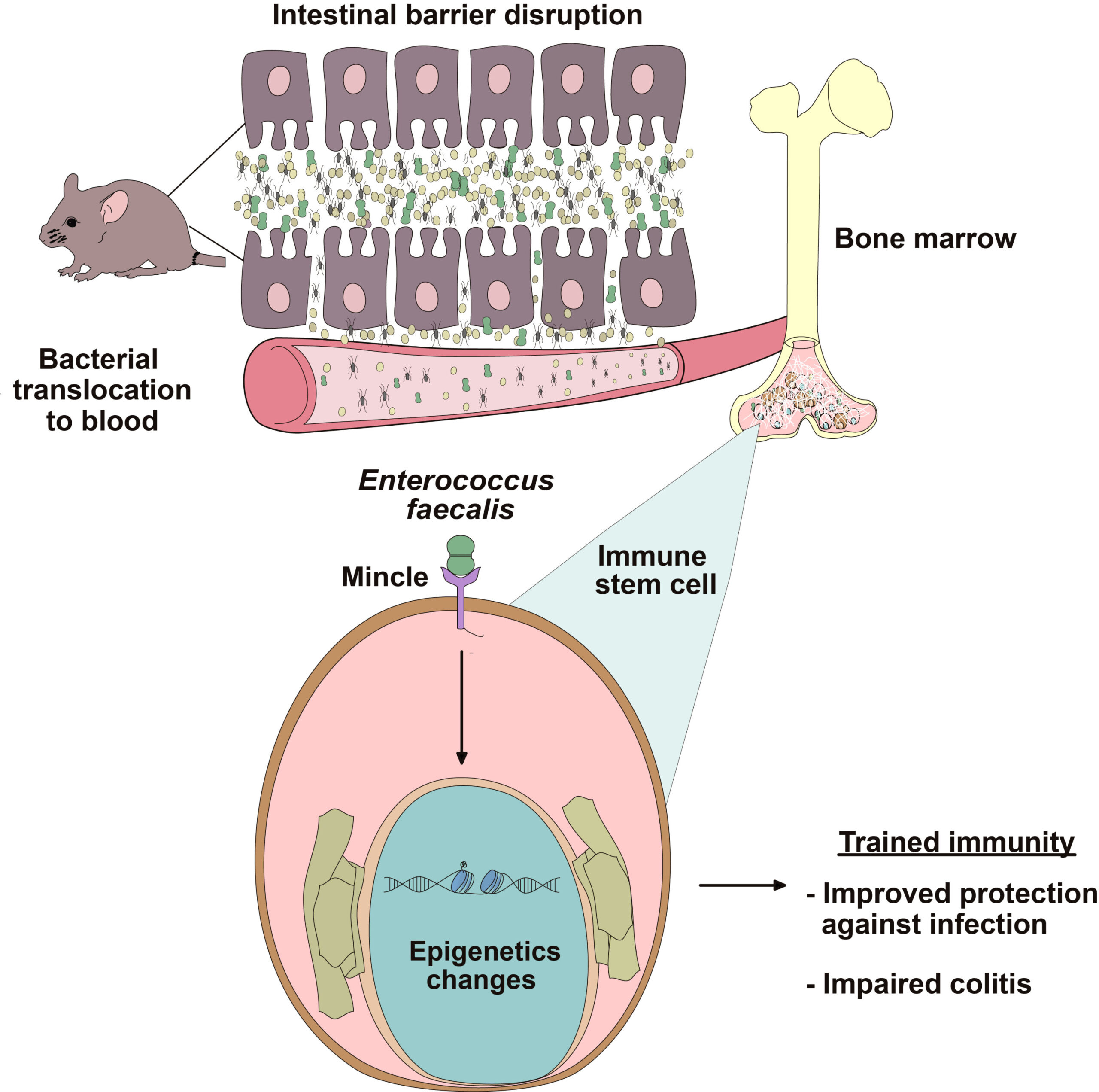
A groundbreaking study conducted by David Sancho at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) in Madrid has unveiled the role of intestinal permeability in inducing changes in immune cell behavior. The research emphasizes how increased intestinal permeability leads to the translocation of natural gut bacteria into the bloodstream, where they influence immune cell development in the bone marrow. This interaction induces epigenetic changes, reprogramming immune cells to become “trained,” enhancing their ability to respond to future infections.
Published in the journal Immunity, this research provides valuable insights into the molecular underpinnings of trained immunity—a previously underexplored phenomenon where innate immune cells “remember” past encounters with pathogens. What makes this discovery particularly significant is the new role of the protein Mincle, expressed in cells of the innate immune system. Sancho’s work shows that Mincle is crucial for detecting translocated bacteria and triggering epigenetic modifications in the hematopoietic precursors (cells that give rise to immune cells) in the bone marrow.
Though the ability of trained immunity to strengthen the immune system’s defense is advantageous, especially for fighting infections, the study also highlights a less desirable outcome. The enhanced immune response fueled by this mechanism can, under certain circumstances, exacerbate inflammatory diseases. Among the conditions linked to this process are cardiovascular diseases (such as atherosclerosis) and neurodegenerative disorders. The findings shed light on the complex interaction between gut health, immune system function, and systemic inflammation, opening new avenues for preventing chronic diseases.
Trained Immunity and Gut Microbes
In simple terms, trained immunity refers to the phenomenon by which innate immune cells—such as macrophages—become more responsive to infections after exposure to a pathogen. This enhanced response, unlike adaptive immunity, is not pathogen-specific but instead reflects a generalized readiness to respond to future infections more effectively.
Until recently, the concept of “immune memory” was mainly attributed to the adaptive immune system, which produces highly specific responses against pathogens based on prior exposure. By contrast, the innate immune system, which constitutes the body’s first line of defense, was long thought to operate without memory, responding to infections in a manner that does not change over time.
However, Sancho’s research challenges this notion. Through his study, it has now been established that innate immunity can indeed “learn” from past exposure, adapting to provide more effective protection against subsequent, even unrelated, infections. Notably, this memory-like effect is not coded in the genetic sequence but occurs through epigenetic changes—modifications that alter gene activity without altering the DNA sequence itself.
These epigenetic modifications affect the functionality of immune cells, making them primed for a stronger and faster response in future encounters with pathogens. It’s a vital mechanism that allows the immune system to be more efficient in fighting off infections.
The study identifies the presence of the bacterium Enterococcus faecalis in bone marrow as a key player in the process. E. faecalis is a Gram-positive bacterium commonly found in the human gastrointestinal tract. Though harmless under normal conditions, this bacterium can cross the intestinal barrier when its integrity is compromised, a situation exacerbated by factors like poor diet or chronic stress.
Once inside the bone marrow, E. faecalis interacts with Mincle on the surface of hematopoietic stem cells, triggering epigenetic changes that alter immune cell behavior, especially in macrophages. As a result, these cells have an amplified inflammatory capacity. In essence, the body is left with a heightened immune response that prepares it for any future infections.
The Dual Nature of Trained Immunity
While trained immunity offers potential benefits—enhancing the body’s defense against infections—it also has a darker side. An exaggerated immune response, if left unchecked, can worsen conditions associated with chronic inflammation. Sancho notes that cardiovascular diseases, especially atherosclerosis, and neurodegenerative diseases are particularly vulnerable to the negative consequences of trained immunity.
Atherosclerosis, for example, is a condition in which the blood vessels harden due to the buildup of fatty deposits. Chronic inflammation plays a major role in the progression of atherosclerosis, and an overstimulated immune response may accelerate this process. Similarly, inflammation is a key contributor to the development of several neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease.
Sancho and his collaborators have shown that intestinal permeability—the leakage of bacteria from the intestines into the bloodstream—plays a central role in this exacerbated inflammation. When the gut barrier becomes leaky, bacteria like Enterococcus faecalis translocate to systemic sites like the bone marrow, where they initiate trained immunity, priming immune cells for more aggressive responses. While this can be beneficial in fighting infections, the overactive immune response in the context of systemic diseases, especially in the absence of infection, can lead to the worsening of inflammatory diseases.
Mincle: A Key Player in Inflammation
A pivotal protein in this process is Mincle, which is expressed in the innate immune system’s cells. Mincle detects translocated bacteria and activates pathways that induce epigenetic changes in immune cells. Notably, experiments in animal models showed that mice lacking Mincle were resistant to the inflammatory effects typically induced by increased intestinal permeability. Without Mincle, the immune system could not detect translocated bacteria, and therefore, inflammation levels remained lower.
The discovery that blocking Mincle can reduce inflammation associated with trained immunity opens up a new potential therapeutic avenue. Therapeutic strategies aimed at inhibiting Mincle could prove useful in managing diseases where inflammation is at the root cause. Moreover, preventing the translocation of gut bacteria could help maintain a healthy immune system and avoid the systemic inflammation linked to various chronic conditions.
The Role of Lifestyle and Diet
The findings of this study also shed light on the relationship between diet, gut health, and inflammation. A poor diet—especially one high in processed foods—along with excessive alcohol consumption, chronic stress, and the use of certain medications can all compromise the gut’s barrier function. This increases the likelihood of bacterial translocation, leading to the type of systemic inflammation seen in trained immunity.
To mitigate these effects and promote overall health, experts recommend a balanced diet rich in fruits, vegetables, and fiber, which supports the health of the gut barrier. Furthermore, such diets can help maintain a balanced microbiota, which is essential not only for a healthy digestive system but also for regulating the immune response and inflammation levels.
Implications for Disease Prevention and Treatment
This research underscores the complex connection between gut health and systemic diseases, shedding light on the importance of maintaining a healthy intestine to prevent inflammatory disorders and chronic diseases. By targeting the mechanisms that underlie trained immunity, such as the protein Mincle, researchers may be able to develop new treatments aimed at modulating immune responses in diseases like atherosclerosis, arthritis, and neurodegenerative diseases.
Maintaining a balanced, nutrient-rich diet, reducing stress, and promoting gut health could be powerful strategies in preventing the systemic inflammation associated with these diseases. As this research continues to evolve, it holds promise for better understanding how gut bacteria interact with the immune system, the way these interactions influence health, and the ways we can modulate these processes for therapeutic benefit.
Conclusion
The study conducted by David Sancho and his team opens up new possibilities in understanding the relationship between gut bacteria, immune responses, and inflammation. By uncovering the key role of Mincle and the influence of intestinal permeability in driving epigenetic changes in immune cells, this research lays the foundation for further exploration of how we can harness or mitigate the effects of trained immunity.
As we look toward the future, this knowledge could lead to novel approaches in managing immune system-related diseases. It also serves as a reminder of the importance of maintaining a healthy gut through lifestyle choices, particularly diet, in preventing chronic diseases linked to inflammation. Through further research and therapeutic advancements, we may be able to strike a balance between the benefits of trained immunity and the risks associated with its excessive activation.
Reference: Iñaki Robles-Vera et al, Microbiota translocation following intestinal barrier disruption promotes Mincle-mediated training of myeloid progenitors in the bone marrow, Immunity (2025). DOI: 10.1016/j.immuni.2024.12.012