In the quest to mitigate climate change, one of the most promising approaches involves capturing and repurposing the excess carbon dioxide (CO2) in the atmosphere. While CO2 is a major contributor to global warming, it also holds potential as a valuable raw material for various industrial applications. Recent breakthroughs have demonstrated that, under the right conditions, CO2 can be transformed into useful substances like methanol—an important chemical used in the production of plastics, pharmaceuticals, and fuels.
A pioneering study by an international team of researchers led by Professor Wolfgang Schuhmann from Ruhr-Universität Bochum, Germany, and Dr. Felipe Conzuelo from Universidade Nova de Lisboa, Portugal, has made a significant step in this direction. The team has developed a hybrid cascade system that combines two powerful methods—electrocatalysis and biocatalysis—to selectively convert CO2 into methanol. This innovative hybrid process has the potential to make CO2 recycling not only more efficient but also more economically viable. Their research was published in the December 23, 2024 edition of Angewandte Chemie International Edition.
Understanding the Challenges of CO2 Conversion
CO2 is one of the most stable and oxidized forms of carbon, which makes its reduction a challenging process. Converting CO2 into something useful, like methanol, requires multiple reduction steps. Electrocatalysis and biocatalysis are two leading methods used for this task, but each has its advantages and drawbacks.
Electrocatalysis: Powerful but Non-Selective
Electrocatalysis involves using electricity to drive chemical reactions that break down CO2 into various potential products, such as formate, carbon monoxide, methane, and even methanol. This process is highly efficient at initiating the reduction of CO2. However, a major issue with electrocatalysis is that, once the reaction starts, the product formation diverges. This means that while electrocatalysis can efficiently break down CO2, it often produces a mixture of up to 16 different products, not necessarily methanol. This lack of selectivity can make it difficult to control the process and optimize the yield of the desired product—methanol.
Biocatalysis: Selective but Sensitive
On the other hand, biocatalysis uses enzymes, which are natural catalysts that can drive chemical reactions in a highly selective manner. Enzymes like formaldehyde dehydrogenase and alcohol dehydrogenase, for example, can specifically convert intermediates in the reaction pathway to methanol. This selectivity is a significant advantage, as it ensures that only the desired product—methanol—is produced.
However, biocatalysts come with their own set of challenges. They are highly sensitive to environmental conditions and often require additional cofactors to function effectively. In the case of the enzymes mentioned above, they require NAD (nicotinamide adenine dinucleotide) as a cofactor, which is consumed during the reaction. This means that for biocatalysis to be sustainable, the NAD cofactor needs to be continually replenished, adding complexity to the process. Additionally, enzymes can be difficult to handle on a large scale, and their effectiveness can be hindered by harsh reaction conditions.
The Hybrid Cascade: A Novel Approach
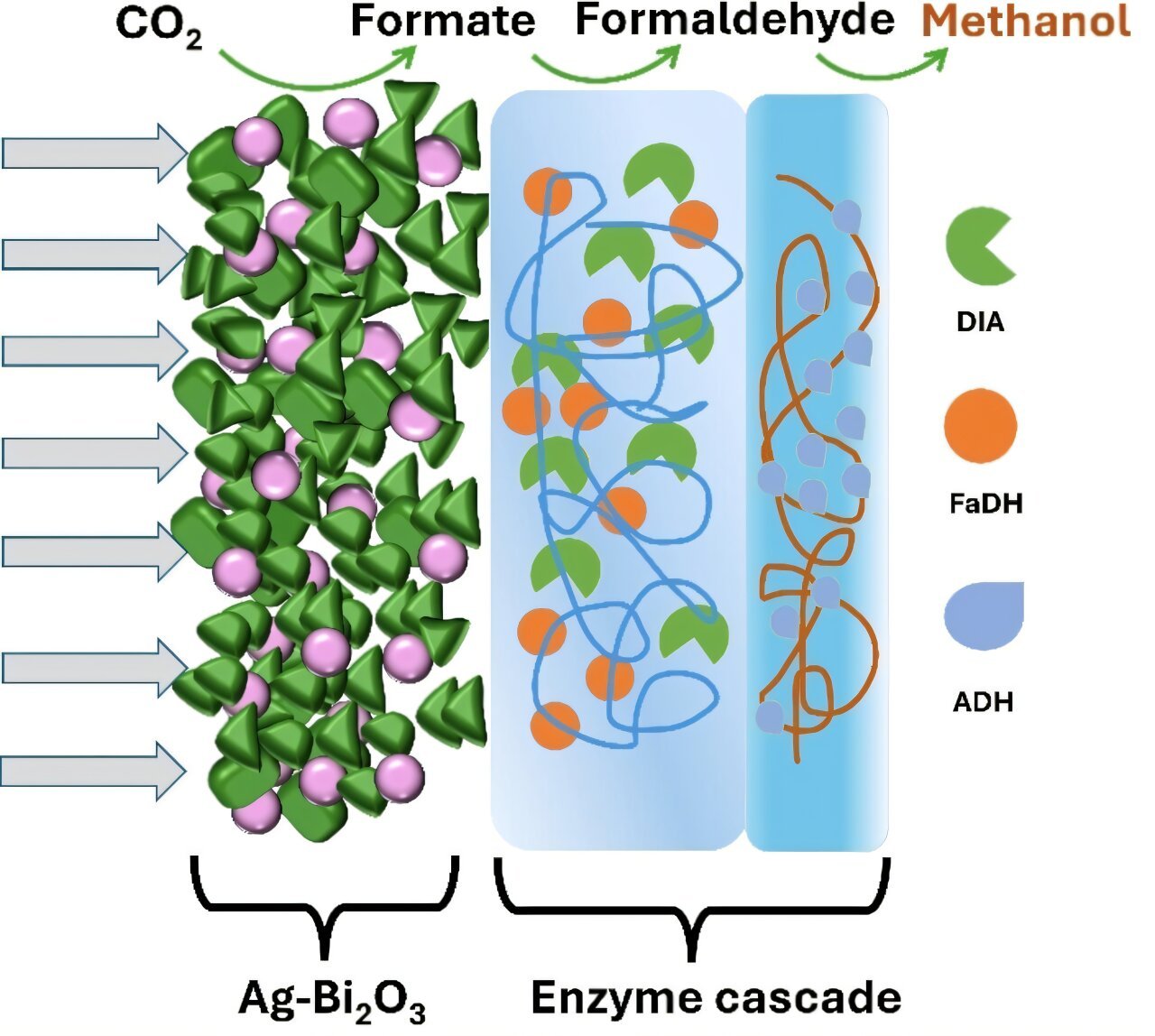
To overcome these limitations, the research team developed a hybrid catalysis cascade that combines the best of both worlds: the efficiency of electrocatalysis and the selectivity of biocatalysis. The hybrid process begins with electrocatalysis, where CO2 is first converted to formate. This is the first critical step in the multistep process, as formate is a key intermediate that can be further reduced to methanol.
The subsequent steps in the cascade are handled by biocatalysts. Once formate is produced, the enzymes formaldehyde dehydrogenase and alcohol dehydrogenase take over, catalyzing the next steps in the reaction. These enzymes convert formate into methanol in a highly selective manner. However, as mentioned earlier, these enzymes require NAD as a cofactor, which is consumed during the reaction.
To address the issue of cofactor regeneration, the team introduced a third enzyme into the system. This enzyme is responsible for regenerating the NAD cofactor, ensuring that the reaction can proceed efficiently and sustainably over time. Once all the steps are completed, the valuable substance methanol is produced.
Why Methanol?
Methanol is a highly sought-after substance in the chemical industry. It serves as a crucial feedstock in the production of a wide range of products, including plastics, paints, adhesives, and solvents. Additionally, methanol is a potential alternative fuel, and its production from CO2 would provide an environmentally friendly and sustainable way to create valuable chemicals while reducing the impact of climate change.
CO2-based methanol production offers several advantages. First, it can help close the carbon loop, where carbon emissions are captured and converted into useful products, reducing the overall amount of CO2 in the atmosphere. Additionally, since methanol is a key raw material for many industries, producing it from CO2 would help reduce the reliance on fossil fuels, offering an alternative, renewable source of carbon.
The Potential of Hybrid Cascades
The hybrid cascade process developed by Schuhmann, Conzuelo, and their team represents a significant step forward in the field of CO2 utilization. This innovative approach successfully marries the advantages of electrocatalysis and biocatalysis, creating a highly efficient and selective method for turning climate-damaging CO2 into valuable methanol. According to Schuhmann, the success of the study “proves that such hybrid cascades are in principle feasible and make complex, multi-step reactions selectively possible.”
The combination of electrocatalysis and biocatalysis in this hybrid cascade offers several key benefits:
- Efficiency: Electrocatalysis kick-starts the process by converting CO2 into formate quickly, while biocatalysis takes over to selectively produce methanol. This integration ensures that the conversion process is efficient at each stage.
- Selectivity: By using biocatalysts, the reaction is highly selective, producing only the desired product—methanol—without the unwanted byproducts that typically arise from electrocatalysis alone.
- Sustainability: The introduction of a cofactor regeneration step ensures that the reaction can continue over an extended period without requiring the addition of fresh cofactors, making the process more sustainable in the long run.
- Scalability: The hybrid cascade system has the potential to be scaled up for industrial applications, where the conversion of CO2 to methanol could become a commercially viable method for producing a valuable chemical while mitigating CO2 emissions.
Looking Forward: Commercialization and Real-World Impact
While the hybrid cascade process is still in the experimental stage, its successful demonstration represents a promising step toward the commercialization of CO2-based methanol production. If further research can refine the process and increase its efficiency, it could become a powerful tool in the fight against climate change.
Governments, industries, and research institutions around the world are increasingly focusing on developing sustainable methods for capturing and repurposing CO2. With global carbon emissions continuing to rise, finding practical ways to recycle CO2 into useful products like methanol is more important than ever. The hybrid cascade developed by Schuhmann, Conzuelo, and their team could play a significant role in achieving this goal, offering an innovative solution to both the problem of climate change and the demand for valuable chemical feedstocks.
As the world continues to explore new ways of tackling climate change, hybrid catalysis systems like this one hold the potential to revolutionize the way we think about CO2 and its role in our industrial processes. By harnessing the power of both electrocatalysis and biocatalysis, researchers have opened the door to a new era of sustainable chemical production, where CO2 can be not only captured but also transformed into valuable products that help build a more sustainable future.
Reference: Panpan Wang et al, Hybrid Enzyme‐Electrocatalyst Cascade Modified Gas‐Diffusion Electrodes for Methanol Formation from Carbon Dioxide, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202422882