Scientists at The Hospital for Sick Children (SickKids) have made a groundbreaking discovery that could pave the way for next-generation treatments for medulloblastoma, the most common form of malignant brain tumor in children. This research, published in Developmental Cell, centers on a key gene called KCNB2, which may offer a new approach to tackling medulloblastoma tumor growth and provide better treatments for patients, potentially revolutionizing how we treat this devastating childhood cancer.
Understanding Tumor-Propagating Cells and Treatment Challenges
Medulloblastoma is an aggressive type of brain tumor that primarily affects young children, and despite advances in treatment, the disease often relapses. One of the main challenges in treating medulloblastoma, as well as other cancers, is the presence of tumor-propagating cells—cells within the tumor that fuel its growth and survival. These special cells can withstand the harsh treatments used in conventional cancer therapy, such as radiation and chemotherapy. As a result, while these treatments may shrink the tumor in the short term, they cannot always eliminate these resilient cells. The tumor can regrow, leading to relapse and more difficult-to-treat conditions.
A Promising Discovery in Potassium Channel Research
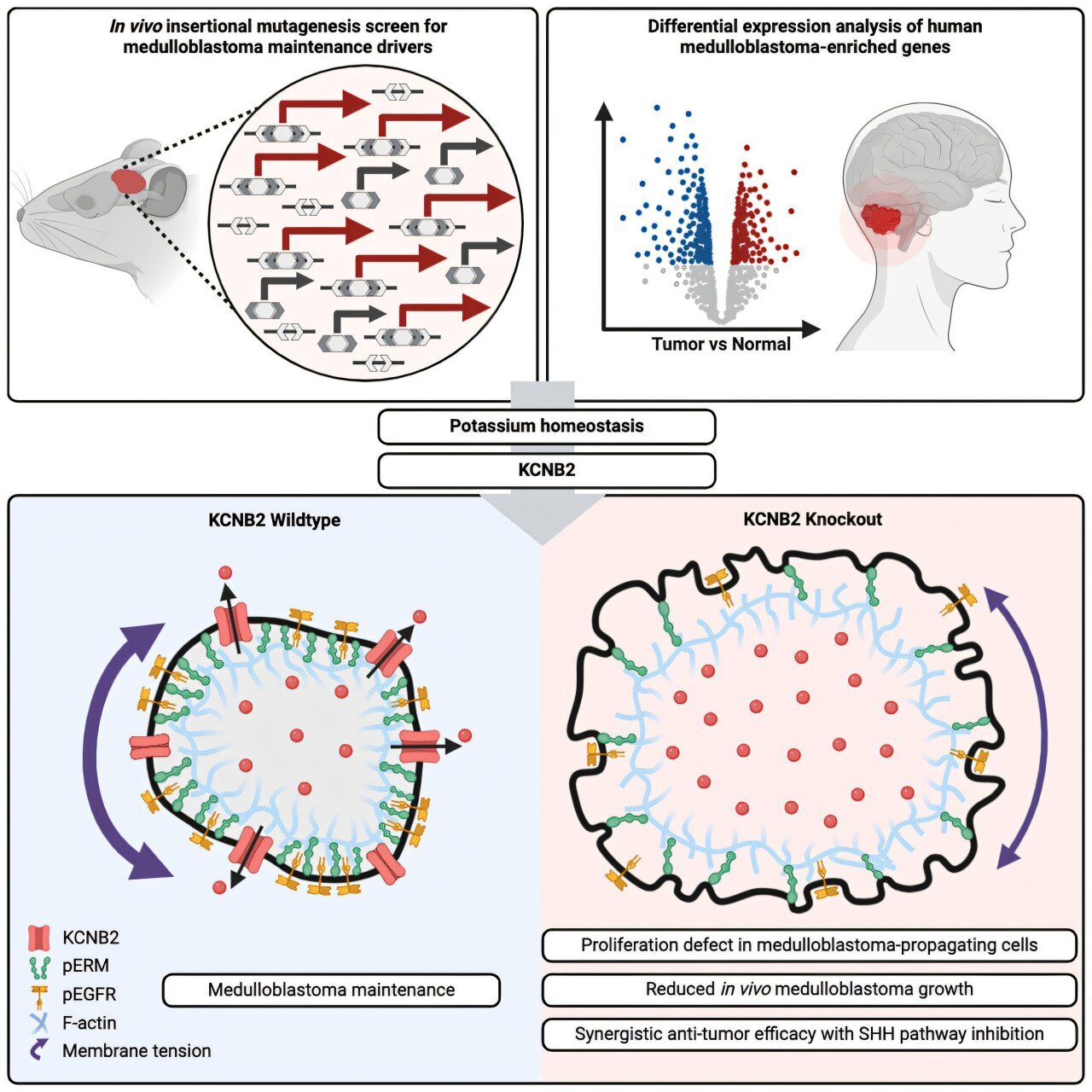
The new study led by Dr. Xi Huang, a senior scientist in the Developmental, Stem Cell & Cancer Biology program at SickKids, has identified a potassium channel gene that appears to play a crucial role in the proliferation of these tumor-propagating cells. According to Dr. Huang, the KCNB2 gene—part of the potassium channel family—could serve as a target for new cancer treatments.
“KCNB2 is a potassium channel, and we have found that by targeting this specific gene, we can disrupt the tumor-propagating cells without harming the surrounding healthy cells,” explains Dr. Huang. “This could enhance existing therapies and transform how we approach this common pediatric brain cancer.”
How the Research Unfolded: A Multi-Step Investigation
The research team, led by Dr. Michael Taylor, a pediatric neurosurgeon at SickKids and expert in cancer research, utilized a genetically engineered preclinical model to explore various genes linked to tumor growth in medulloblastoma. Their investigations revealed a list of potassium channels that seemed to be disproportionately expressed in medulloblastoma tumors, as shown by the tumor transcriptome analysis.
This discovery led to further investigations by Dr. Jerry Fan, the first author of the study, who focused on the role of potassium channels in tumor progression. Fan’s analysis narrowed down the function of the KCNB2 channel in particular. “We found that blocking KCNB2 led to a significant disruption in the integrity of the tumor cells,” Fan explains. “This disruption prevents the tumor-propagating cells from dividing, ultimately inhibiting the tumor from growing and spreading.”
Understanding the Role of Potassium and KCNB2 in Tumor Growth
So, what is the connection between potassium and tumor growth? Potassium ions are crucial for many cellular functions, one of which is maintaining fluid balance inside cells. Think of potassium as essential in regulating cell volume—the right amount of potassium allows the cell to maintain its structural integrity.
However, when KCNB2 is inhibited, this normal function is disrupted. Researchers discovered that the inhibition of this potassium channel led to a dramatic increase in water flow into the tumor cells, causing them to swell and rupture, similar to an overfilled balloon. As the tumor cells expanded and their internal structures broke down, the tumor growth mechanisms that were responsible for fueling medulloblastoma ceased to function.
From Discovery to Potential Treatment: Targeting KCNB2
This discovery is incredibly exciting because it could lead to therapies specifically designed to target tumor-propagating cells while leaving healthy brain cells unaffected. With this new insight into the biology of medulloblastoma, the research team began collaborating with an ion channel drug discovery company to evaluate potential drug candidates that could block the function of KCNB2. Over 30,000 small molecules were screened in hopes of identifying those that could effectively inhibit the KCNB2 channel and ultimately stop tumor growth.
“We have now identified promising molecules that show potential for targeting KCNB2,” says Dr. Huang. “With our research continuing into preclinical testing, we’re eager to see how these molecules work in models that simulate the complexity of human medulloblastoma.”
A New Path Toward Better Therapies
As the research team moves forward with their studies, the next steps involve testing the top candidates in preclinical models to evaluate their efficacy. Dr. Huang and his team hope to identify a molecule that can effectively and safely inhibit the KCNB2 channel, leading to the development of targeted therapies specifically for medulloblastoma patients. Moreover, this research holds promise not just for children with medulloblastoma, but also for patients with other cancers where tumor-propagating cells are a factor.
The development of these targeted therapies is further bolstered by support from the SickKids Industry Partnerships & Commercialization (IP&C) office, which works to ensure that promising discoveries like this one find their way out of the lab and into real-world applications. According to Dr. Huang, this dedicated partnership is essential for bridging the gap between basic science discoveries and clinical treatments.
“Identifying effective molecules to block KCNB2 is a milestone, but our journey doesn’t stop there. Moving this work toward viable treatments for patients will require thorough clinical testing,” says Dr. Huang. “Thanks to the support of SickKids’ IP&C office, we’re optimistic about getting these therapies into the hands of medical professionals and transforming care for young patients fighting medulloblastoma.”
Looking Ahead: Transforming Medulloblastoma Treatment
This research shines a bright light on the potential of personalized and targeted therapies that can offer better outcomes for pediatric brain cancer patients. By focusing on the underlying mechanisms of tumor growth rather than just attempting to kill tumor cells, these types of therapies are more likely to improve patient survival and quality of life while reducing the damaging side effects of traditional treatments like chemotherapy.
As medulloblastoma continues to challenge medical science, the identification of the KCNB2 gene as a key driver of tumor growth opens up new avenues for therapeutic strategies. This research not only highlights the remarkable promise of ion channel-targeting approaches, but also reinforces the importance of innovative, precision medicine to combat the devastating effects of cancer in children.
With further investigation and validation, this breakthrough could eventually result in therapies that significantly change the outlook for children diagnosed with medulloblastoma. The innovative approach presented by Dr. Huang and his team could be one of the keys to unlocking new, highly effective treatments that may one day offer a cure for this challenging childhood brain cancer.
Conclusion
The discovery of the KCNB2 gene as a crucial player in medulloblastoma growth marks a significant milestone in pediatric cancer research. By targeting tumor-propagating cells through the inhibition of this potassium channel, the research team has opened the door to developing more precise, effective therapies for this common but deadly brain cancer. With further preclinical testing and clinical validation, this research could soon transform how medulloblastoma is treated and significantly improve the prognosis for young patients.
Reference: Jerry J. Fan et al, A forward genetic screen identifies potassium channel essentiality in SHH medulloblastoma maintenance, Developmental Cell (2025). DOI: 10.1016/j.devcel.2025.01.001