The energy transition — the shift from fossil fuel-based power generation to renewable energy sources such as wind and solar — presents an array of technical and logistical challenges. One of the most significant obstacles is the storage of electricity produced by renewable sources when it is abundant but not immediately needed. As energy production fluctuates throughout the day or year, a reliable storage system is essential to ensure a steady supply when conditions change. While there are various storage solutions in development, one breakthrough may lie in an unexpected place: lavender oil.
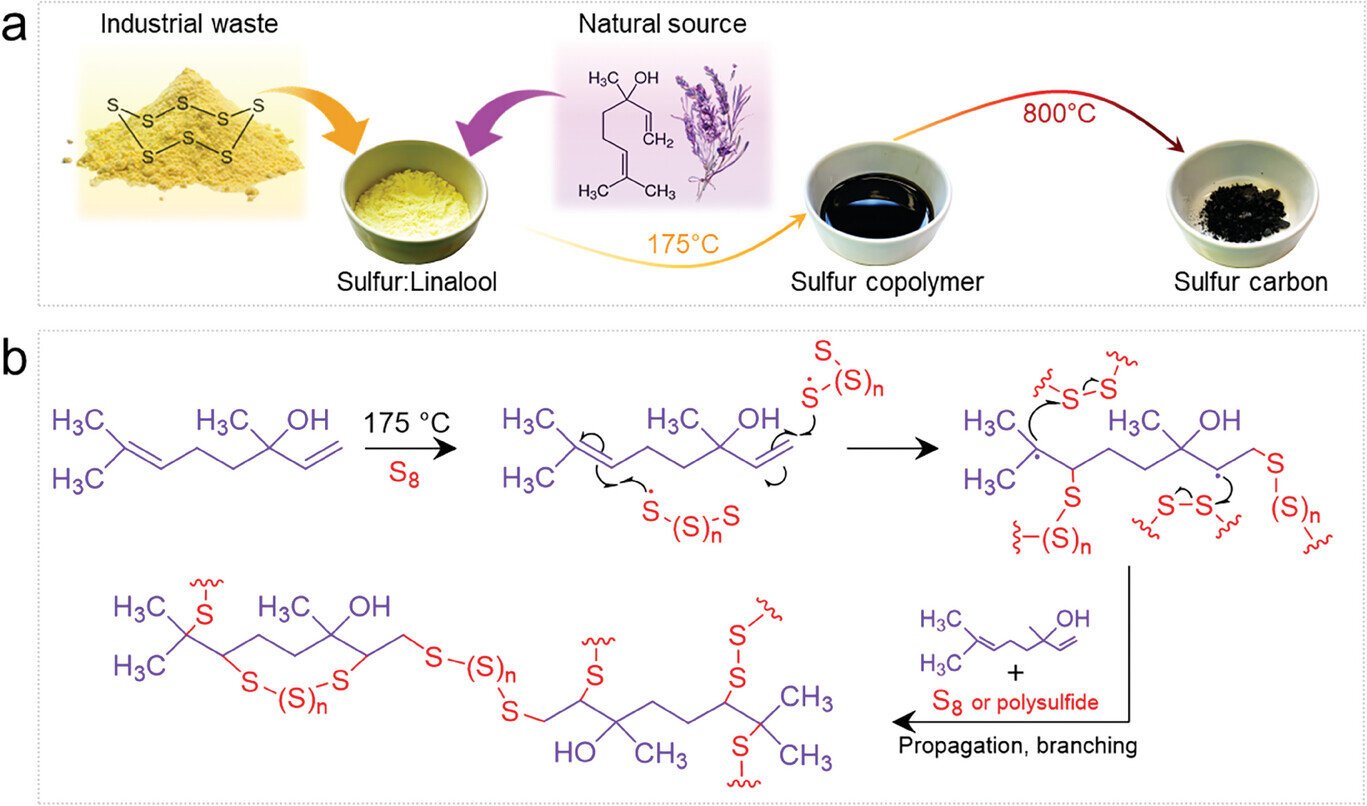
Researchers from the Max Planck Institute of Colloids and Interfaces have developed a new material using linalool, the main component of lavender oil, and sulfur to enhance the performance of sodium-sulfur batteries. This innovation could improve the durability and energy density of these batteries, offering a promising solution for large-scale stationary energy storage. The ability to store and manage electricity from renewable sources efficiently is one of the key pillars in the transition to a clean energy future, and this lavender-derived material might be a crucial element in addressing that challenge.
The Need for Effective Energy Storage Solutions
As renewable energy sources like wind and solar continue to gain traction in the global energy mix, one critical question arises: How can we store electricity when it’s plentiful, so that it can be used when production dips? The sun doesn’t always shine, and the wind doesn’t always blow — creating significant fluctuations in energy availability. Effective energy storage systems are required to address these gaps, providing power when there’s a shortfall in renewable production.
Battery technology, in particular, has become a focal point in this pursuit. Sodium-sulfur batteries, which use sulfur and sodium to store energy, are gaining attention due to their advantages over the more commonly used lithium-ion batteries. Sodium and sulfur are far more abundant and environmentally friendly than the metals required for lithium-ion batteries, such as lithium and cobalt, whose mining processes can cause significant environmental damage and social issues in the regions where they are extracted.
However, sodium-sulfur batteries are not without their drawbacks. These batteries tend to have lower energy density compared to lithium-ion batteries, meaning they store less energy relative to their weight. Moreover, their durability has been a significant concern, with capacity deteriorating quickly after a relatively small number of charging cycles.
Innovative Solution: Lavender Oil and Sulfur in Sodium-Sulfur Batteries
Now, a team at the Max Planck Institute of Colloids and Interfaces has developed an innovative solution that could extend the lifespan and increase the energy density of sodium-sulfur batteries. By utilizing linalool, a compound found in lavender oil, the researchers have managed to create a material that can stabilize the sulfur in sodium-sulfur batteries, preventing common issues that lead to battery failure.
According to Paolo Giusto, group leader at the Max Planck Institute, the team’s breakthrough is exciting because it uses something as everyday as lavender oil to improve energy storage technology. “It’s fascinating to design future batteries with something that grows in our gardens,” Giusto says.
The team’s work, which has been published in the journal Small, centers on addressing one of the most significant issues with sodium-sulfur batteries: sulfur shuttling. In traditional sodium-sulfur batteries, polysulfides — byproducts formed during the battery’s discharge cycle — can migrate from the cathode to the anode. Once there, the polysulfides react with the anode material, leading to a reduction in the battery’s efficiency and lifespan. This issue is one of the reasons why sodium-sulfur batteries typically experience rapid performance degradation after only a few charging cycles.
How Lavender Oil Helps: The Role of Linalool
The key to the team’s breakthrough is the combination of linalool and sulfur to create a stable, dense nanomaterial. This material, which features nanostructures with pores that are about 100,000 times smaller than a human hair, acts as a carbon cage to trap the polysulfides formed at the cathode. This nanocage prevents the polysulfides from migrating to the anode, where they could otherwise degrade the battery.
However, the novel material doesn’t just lock the polysulfides in place — it also allows the small sodium ions to move freely in and out of the nanostructures during the battery’s charging and discharging cycles. This means the battery can still function effectively, but with significantly improved durability and storage capacity.
As a result of this innovation, the sodium-sulfur batteries tested by the researchers retained more than 80% of their original charging capacity after 1,500 cycles — a considerable improvement over traditional sodium-sulfur batteries, which typically see a significant drop in capacity much earlier.
Improved Durability and Higher Energy Density
The introduction of the carbon nanovessels enclosing the sulfur offers several benefits. First, it drastically improves the durability of sodium-sulfur batteries, helping them last much longer than traditional versions. The material also enhances the energy density of the batteries, meaning they can store more energy in a given amount of space. The batteries constructed with this new material were able to deliver more than 600 mAh/g, a significant increase compared to current sodium-sulfur batteries.
By stabilizing the sulfur in the battery and preventing the polysulfides from damaging the anode, this new approach makes sodium-sulfur batteries not only more durable but also more efficient at storing energy. The material is thus a crucial step toward developing a viable and sustainable alternative to lithium-ion batteries, particularly for large-scale, stationary energy storage.
A Creative Look at Nature’s Solutions
What makes this development even more remarkable is that it draws inspiration from nature. Linalool, a compound found in lavender, is an organic material that is abundant, biodegradable, and safe to handle. This creative use of a naturally occurring substance to solve a technological problem highlights the importance of bio-inspired design in modern engineering.
As Evgeny Senokos, one of the researchers behind the project, explains, “We create a stable and dense nanomaterial from linalool and sulfur and thus obtain batteries that are more durable and have a higher energy density than today’s sodium-sulfur batteries.” The use of linalool in this context demonstrates the potential for sustainability in the development of future technologies, where solutions from the natural world are incorporated into human-made innovations.
Looking Ahead: From Lab to Real-World Applications
The development of more efficient, durable, and sustainable sodium-sulfur batteries could have far-reaching implications for the energy transition. As Paolo Giusto points out, “By taking a creative look at nature, we are finding solutions to many of the challenges posed by the energy transition. I am confident that our development will attract increasing attention in the near future and enable us to make the leap of this technology from laboratory to practice.”
This technology has the potential to transform the way we store energy and could play a significant role in addressing the intermittency issues associated with renewable energy sources. The ability to store large amounts of energy in an environmentally friendly and sustainable manner would make it easier to integrate wind and solar power into the global energy grid, reducing reliance on fossil fuels and helping to mitigate climate change.
In conclusion, the use of lavender oil as a key component in the development of sodium-sulfur batteries represents an exciting and promising breakthrough in the quest for more effective and sustainable energy storage solutions. By combining cutting-edge scientific research with bio-inspired design, the Max Planck team has paved the way for batteries that could be essential to the success of the energy transition. The next challenge is to scale this technology up and bring it from the lab into practical, real-world applications — an endeavor that could have a profound impact on our energy future.
Reference: Evgeny Senokos et al, Sustainable Sulfur‐Carbon Hybrids for Efficient Sulfur Redox Conversions in Nanoconfined Spaces, Small (2024). DOI: 10.1002/smll.202407300