When children receive their second dose of the measles-mumps-rubella (MMR) vaccine around the time they start kindergarten, they acquire long-lasting protection against all three viruses, often extending for their entire lives. In contrast, the effectiveness of an influenza vaccine administered in October typically wanes by the following spring. This stark difference in the durability of vaccine-induced immunity has puzzled scientists for decades. The question of why some vaccines confer long-term protection while others offer only temporary immunity remains one of the most compelling mysteries in immunology.
Recently, groundbreaking research led by scientists at Stanford Medicine has provided significant insights into this phenomenon. The study identified a surprising role played by a type of blood cell called megakaryocytes, which are traditionally known for their involvement in blood clotting. The findings suggest that these cells may also play a crucial role in determining how long a vaccine can stimulate the production of protective antibodies.
The research, spearheaded by Bali Pulendran, Ph.D., a professor of microbiology and immunology, sheds light on the molecular mechanisms underlying vaccine durability. “Our study defines a molecular signature in the blood, induced within a few days of vaccination, that predicts the durability of vaccine responses,” Pulendran stated. This discovery not only unravels a long-standing question in vaccine science but also provides a potential pathway for developing vaccines that could offer more lasting immunity.
In a prior study conducted in 2022, Pulendran and his team identified a “universal signature” capable of predicting an early antibody response to various vaccines. However, this earlier research did not address the longevity of these antibody responses. The new findings, published in Nature Immunology, go a step further by pinpointing a molecular signature associated with long-term immunity.
The Stanford researchers initially focused on an experimental H5N1 bird flu vaccine administered with an adjuvant—a substance added to vaccines to enhance immune responses. Fifty healthy volunteers participated in the study, receiving either two doses of the vaccine with the adjuvant or two doses without it. Blood samples were collected from these participants at multiple intervals over 100 days following vaccination. Using advanced machine-learning algorithms, the researchers analyzed patterns in the data, including genes, proteins, and antibodies present in the blood samples.
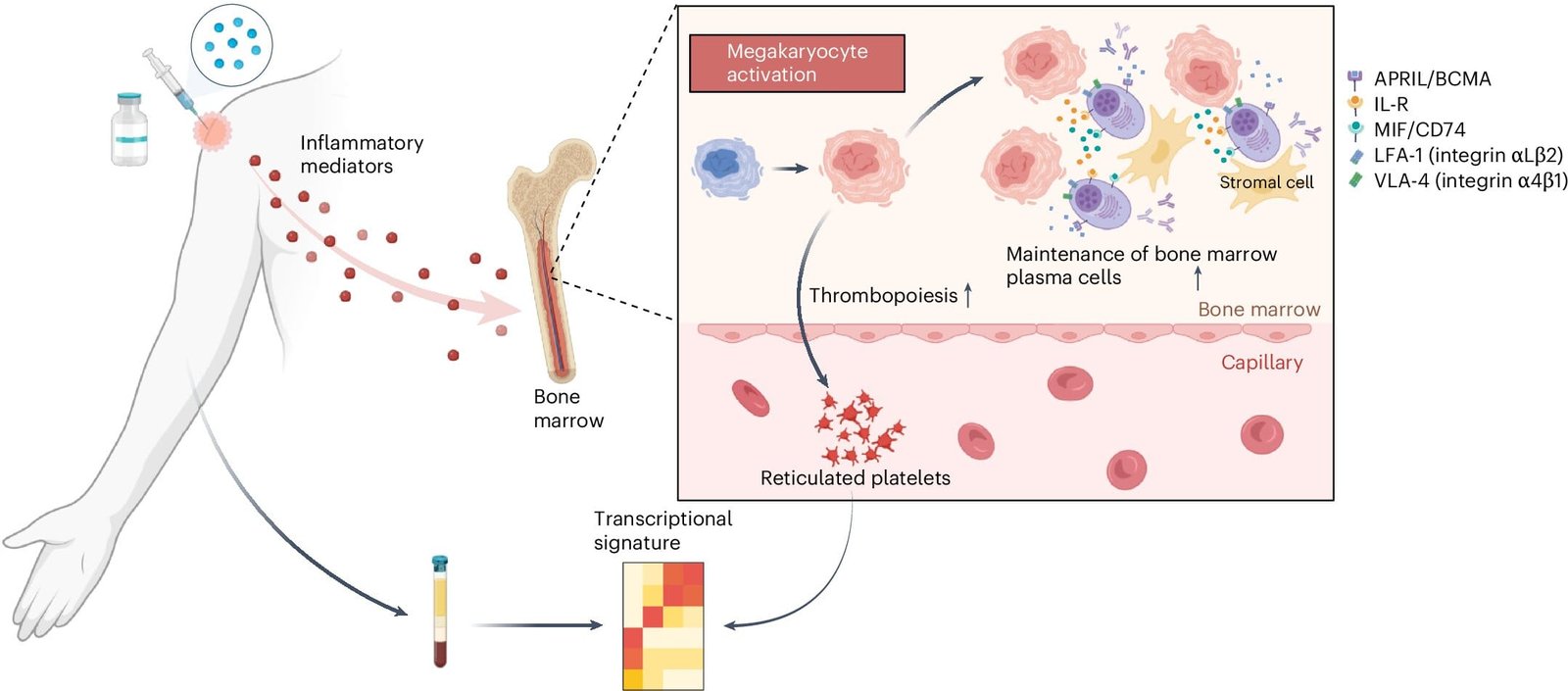
The analysis revealed a molecular signature that appeared in the blood shortly after vaccination and correlated with the strength and duration of antibody responses. Interestingly, this signature was primarily linked to tiny RNA fragments found in platelets. Platelets, small blood components essential for clot formation, are derived from megakaryocytes—large cells located in the bone marrow. When platelets break off from megakaryocytes, they carry with them small pieces of RNA from their parent cells. These RNA fragments served as markers for megakaryocyte activity, providing researchers with indirect insights into the processes occurring in the bone marrow.
“What we learned was that the platelets are a bellwether for what is happening with megakaryocytes in the bone marrow,” Pulendran explained. This observation suggests that megakaryocytes might play a previously unrecognized role in shaping the immune response to vaccines.
To test this hypothesis, the researchers conducted experiments in mice. They administered the bird flu vaccine along with thrombopoietin, a drug that increases the number of activated megakaryocytes in the bone marrow. The results were striking: mice that received thrombopoietin exhibited a sixfold increase in antibody levels two months after vaccination. Additional experiments revealed that activated megakaryocytes produce molecules that support the survival of plasma cells—specialized cells responsible for producing antibodies. Blocking these molecules led to reduced survival of plasma cells, further underscoring the role of megakaryocytes in sustaining long-term antibody production.
The researchers extended their investigation to other vaccines, including those targeting seasonal influenza, yellow fever, malaria, and COVID-19. By analyzing data from 244 individuals, they found that the molecular signature linked to megakaryocyte activity consistently predicted longer-lasting antibody responses across different vaccine types. This discovery suggests that megakaryocyte activation is a general mechanism influencing vaccine durability.
The implications of this research are profound. By understanding the factors that contribute to long-lasting immunity, scientists could potentially design vaccines that more effectively activate megakaryocytes, leading to improved durability of immune responses. Additionally, the findings could pave the way for personalized vaccination strategies. For instance, a simple diagnostic test could be developed to measure the molecular signature identified in this study. Such a test could predict how long a vaccine’s protection is likely to last for an individual, enabling tailored vaccination schedules and reducing the need for frequent booster doses.
“We could develop a simple PCR assay—a vaccine chip—that measures gene expression levels in the blood just a few days after someone is vaccinated,” Pulendran said. This approach could revolutionize vaccine development and evaluation by allowing researchers to quickly assess a vaccine’s durability without waiting months or years for long-term data. Furthermore, it could help identify individuals who might require additional booster doses to maintain adequate immunity.
While this research represents a significant step forward, Pulendran emphasized that vaccine durability is influenced by a complex interplay of factors. Megakaryocytes are likely just one piece of the puzzle. Other components of the immune system, as well as individual genetic and environmental factors, may also play critical roles in determining the longevity of vaccine-induced immunity.
The study involved contributions from an extensive network of collaborators, including researchers from the University of Cincinnati, Emory Vaccine Center, University of California, San Diego, GSK Belgium, Hospital Israelita Albert Einstein, Jackson Laboratory, the Food and Drug Administration, Icahn School of Medicine at Mount Sinai, Emory University School of Medicine, the National Institutes of Health, and NYU Grossman School of Medicine. This multidisciplinary effort underscores the complexity and collaborative nature of vaccine research.
Reference: Mario Cortese et al. System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans, Nature Immunology (2025). DOI: 10.1038/s41590-024-02036-z. www.nature.com/articles/s41590-024-02036-z
Think this is important? Spread the knowledge! Share now.