Just like your body relies on its skeleton for support and resilience, each cell in your body has its own internal framework called the cytoskeleton. This cellular skeleton not only provides structural integrity but also plays a vital role in cell division, shape maintenance, and response to mechanical forces. To delve deeper into how real cells work, particularly for the purposes of drug development and disease research, scientists often create artificial cells in laboratory settings. However, many of these artificial cells lack a cytoskeleton, which limits their usefulness in studying cellular responses to mechanical forces. Researchers at Eindhoven University of Technology (TU/e) have made significant progress in overcoming this limitation by designing a polymer-based network for artificial cells that mimics a natural cytoskeleton, allowing for more accurate studies of how cells respond to forces.
Currently, scientists are using artificial cells to mimic natural cells as a means of understanding life processes. This might seem counterintuitive at first, as studying living cells directly might appear more straightforward. However, there is solid logic behind this approach. Artificial cells provide a controlled environment for studying biological processes, free from the complexities and variability inherent to living cells. They enable researchers to dissect cellular mechanisms step-by-step, testing how specific molecules influence cell functions. This kind of research has applications ranging from more efficient drug screening processes and advanced drug delivery technologies to tissue regeneration techniques.
Traditionally, researchers focused on incorporating key biological functions into artificial cells. For example, they sought to replicate basic cellular activities like communication with other cells and movement. These advancements have brought artificial cells closer to natural cells in terms of functionality. But there remained a significant gap when it came to studying the mechanical properties of cells, particularly how they respond to physical forces—a gap largely due to the absence of a cytoskeleton in artificial cells.
To understand why including a cytoskeleton is so crucial, consider the role it plays in living cells. While the human skeleton is made up of bones, a cytoskeleton is a network of protein filaments. It comprises microtubules formed from tubulin proteins and microfilaments made of actin. These filaments provide a scaffolding-like structure that gives cells their shape and enables them to resist external forces, much like bones support the human body. Additionally, the cytoskeleton facilitates processes such as intracellular transport and changes in cell shape. While natural cells rely on these dynamic networks, replicating them in artificial cells has long been a challenge.
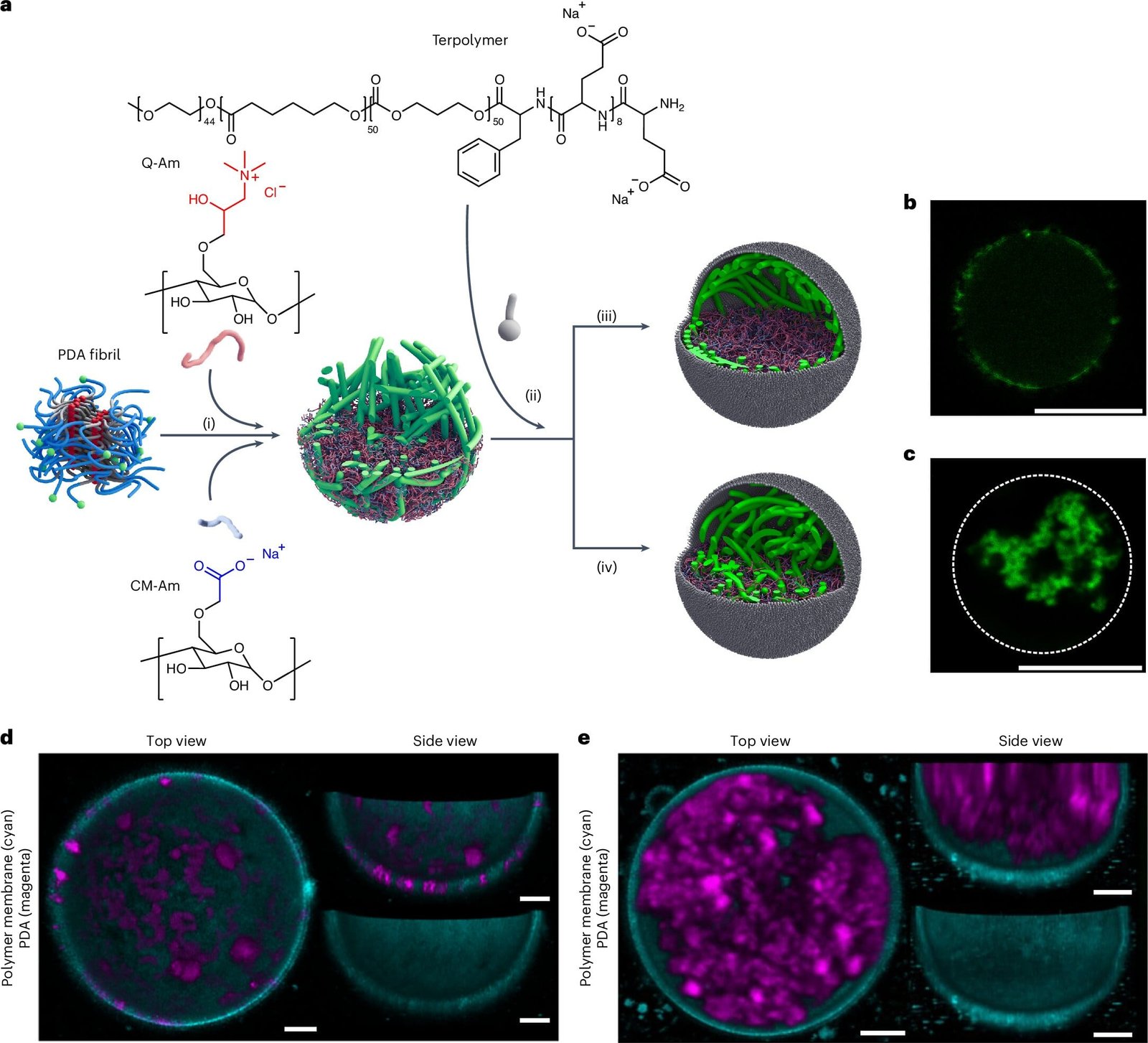
To address this, a team of researchers at TU/e, collaborating with experts at the Max Planck Institute in Germany, designed an artificial cytoskeleton for lab-grown cells using polydiacetylene-based (PDA) polymers. PDA was chosen because it closely mimics the properties of a natural cytoskeleton. It bundles into fibrous structures similar in size to the filaments in living cells and exhibits flexibility, allowing it to deform under mechanical stress. These characteristics make PDA an ideal material for constructing a synthetic cytoskeleton.
Creating a cytoskeleton within artificial cells was only part of the challenge. The next critical step was to test whether this artificial framework could replicate the mechanical properties of its natural counterpart. To do this, the researchers employed a cutting-edge technique called real-time deformability cytometry (RT-DC). This method allowed them to observe in real time how artificial cells deformed as they were subjected to mechanical forces by being pushed through narrow channels. The results were promising: artificial cells equipped with a PDA-based cytoskeleton showed reduced deformation compared to those without, indicating increased stiffness. Moreover, their stiffness—quantified using a measure called Young’s modulus—was similar to that of certain human cells.
These findings mark an important breakthrough. By incorporating a cytoskeleton into artificial cells, researchers can now account for both chemical and mechanical signals in their studies. This is especially relevant when artificial cells interact with living cells, such as in experiments designed to modulate immune cell behavior. The ability to integrate mechanical signals opens up new possibilities for understanding complex cell-cell interactions and the mechanics of cellular environments.
Looking ahead, the research team plans to explore how these mechanically enhanced artificial cells behave when interacting with natural cells. They aim to investigate the combined functional and mechanical properties of their system, which could prove significant for applications where living cells play an integral role. These studies could advance our understanding of biological processes, contribute to the development of new therapeutic strategies, and refine tissue engineering techniques.
This research also reflects a story of collaboration and personal connection. The project was undertaken in partnership with the company SyMO-Chem, whose late co-founder, Henk Janssen, was a close collaborator and a driving force behind the investigation. His expertise in synthetic organic chemistry and mentorship played a pivotal role in shaping the study. In tribute to his contributions and memory, the researchers dedicated their publication in Nature Chemistry to him, recognizing his lasting impact on their work.
This study underscores the potential of artificial cells as tools for scientific discovery. By bridging the gap between artificial and natural systems, the researchers have not only enhanced our understanding of cellular mechanics but also laid the groundwork for future innovations in biomedical engineering and biotechnology. The inclusion of a cytoskeleton in artificial cells represents a critical step toward creating more lifelike models for studying the intricacies of life at a cellular level.
Reference: Sebastian Novosedlik et al, Cytoskeleton-functionalized synthetic cells with life-like mechanical features and regulated membrane dynamicity, Nature Chemistry (2025). DOI: 10.1038/s41557-024-01697-5
Think this is important? Spread the knowledge! Share now.