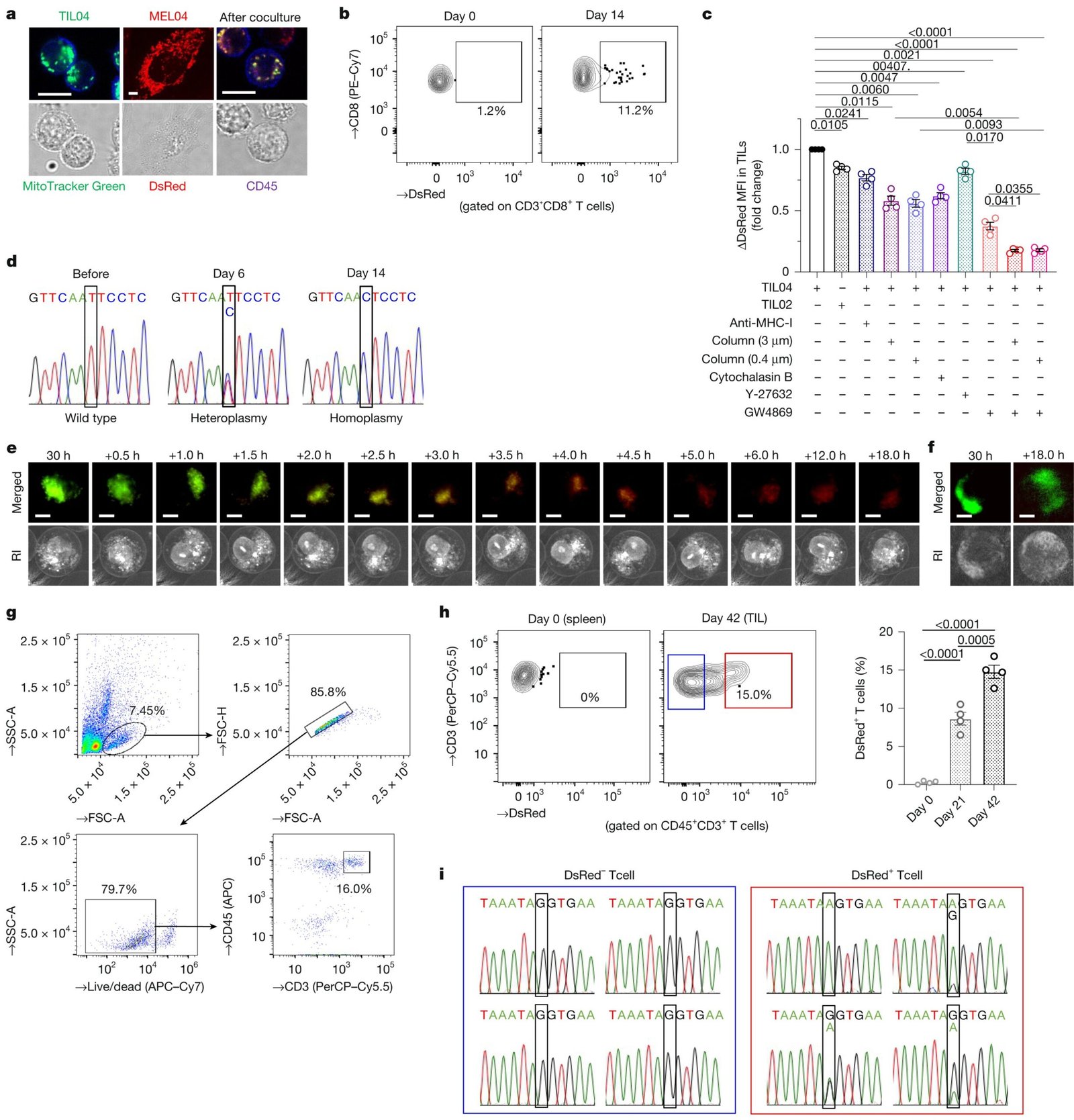
Recent research led by the Chiba Cancer Center Research Institute in Japan has uncovered a novel and unexpected way that cancer cells manage to evade the immune system, potentially explaining why some immunotherapies are successful in certain patients but not in others. The findings, published in Nature under the study titled “Immune evasion through mitochondrial transfer in the tumor microenvironment,” highlight a remarkable mechanism where cancer cells transfer their own faulty mitochondrial DNA (mtDNA) into T-cells, the immune system’s frontline defenders. This action weakens the immune cells, rendering them less effective in fighting tumors.
The Mysterious Transfer of Mitochondrial DNA
Cancer cells have long been known to adopt strategies to evade immune detection, but the discovery of mitochondrial transfer adds a new layer of complexity. The study demonstrated how cancer cells in both melanoma and non-small cell lung cancer (NSCLC) interact with tumor-infiltrating lymphocytes (TILs)—a class of immune cells typically designed to target and eliminate tumor cells. These interactions revealed that cancer cells could transfer damaged mitochondria to T-cells, which are crucial for immune responses against cancer.
Mitochondria are often referred to as the “powerhouses” of the cell, responsible for generating the majority of a cell’s energy through processes such as oxidative phosphorylation. T-cells, which play a significant role in recognizing and fighting tumors, require substantial amounts of energy to function effectively. When cancer cells transfer mitochondria with defective DNA to these T-cells, they effectively reduce the T-cells’ energy capabilities. The defected mitochondria impede normal energy production in the T-cells, leading to their dysfunction and eventual exhaustion, thus weakening the immune system’s ability to fight the tumor effectively.
How the Transfer Works: Tunneling Nanotubes and Extracellular Vesicles
The mechanism by which cancer cells transfer these defective mitochondria into T-cells has been identified through innovative approaches. The study points out two major ways this mitochondrial transfer occurs:
- Tunneling Nanotubes (TNTs): These are narrow, tube-like structures that extend from one cell to another. Cancer cells use TNTs to create tiny passages, allowing the transfer of mitochondria directly into T-cells. Once inside, the mitochondria contribute to the weakening of the immune cell’s function.
- Extracellular Vesicles: These are membrane-bound bubbles formed and released by cancer cells, containing not only mitochondria but also other molecules, including the faulty mtDNA. The vesicles are taken up by T-cells and transfer the defective mitochondria, compromising the cells’ ability to produce the energy needed for optimal immune response.
Through these methods, the faulty mitochondria from cancer cells replace the healthy mitochondria in T-cells—a process that operates in reverse compared to typical mitochondrial turnover, where damaged mitochondria are replaced by healthy ones. Furthermore, cancer cells shield these damaged mitochondria by attaching protective molecules to them, making it difficult for T-cells to recognize and degrade the defective mitochondria.
Impact on Immune Function and Cancer Treatment
The discovery that cancer cells can transfer defective mitochondria into immune cells helps explain why immunotherapies, such as immune checkpoint inhibitors (ICIs), work for some patients but not for others. ICIs have been groundbreaking in treating various cancers by unleashing the body’s immune system to better fight the tumor. However, the effectiveness of these therapies has been inconsistent, particularly in cancers that harbor more mitochondrial mutations. The researchers discovered that tumors with increased mitochondrial mutations were less likely to respond to immune checkpoint inhibitors, likely because the mitochondrial defects already present in the T-cells hinder their ability to respond effectively to cancer.
This knowledge sheds light on how a lack of energy production in T-cells could impair their immune functions, thus rendering immune checkpoint inhibitors less effective. The discovery provides important insight into why some patients do not benefit from these otherwise promising cancer therapies.
A Potential Solution: Blocking the Transfer of Mitochondria
The researchers didn’t just identify this immune-evasion tactic; they also explored ways to counteract it. Using a compound known as GW4869, which inhibits the release of extracellular vesicles, the team successfully blocked the mitochondrial transfer from cancer cells to T-cells. The use of GW4869 in preclinical models showed promising results:
- T-cells treated with the compound demonstrated improved energy production.
- These T-cells showed fewer signs of exhaustion.
- The T-cells exhibited a better capacity to perform their immune functions.
As a result, the application of GW4869 helped restore the effectiveness of immune checkpoint inhibitors, particularly in tumors that exhibited significant mitochondrial transfer. This suggests that targeting the process by which cancer cells transfer defective mitochondria to T-cells could be a new avenue for enhancing the effectiveness of immunotherapy in patients whose tumors have a higher load of mitochondrial mutations.
Implications for Future Cancer Therapies
The findings of this research carry significant implications for the field of cancer therapy. Understanding that mitochondrial transfer plays a key role in immune evasion offers a more detailed understanding of why certain treatments are ineffective. It also introduces the possibility of counteracting this evasive tactic by blocking mitochondrial transfer. By inhibiting the methods cancer cells use to corrupt the immune cells (such as TNTs and extracellular vesicles), scientists may enhance the function of T-cells, improving the outcomes of immunotherapy and other cancer treatments.
Furthermore, targeting the machinery involved in mitochondrial transfer could become an additional strategy for combination cancer therapies, allowing for more personalized and precise approaches. Not all patients respond to immune checkpoint inhibitors in the same way, but this research opens up the potential to use specific interventions to address the unique immune evasion strategies employed by individual tumors.
A New Layer to Cancer’s Immune Evasion Tactics
Typically, research in cancer immunotherapy advances incrementally, with each discovery serving as a stepping stone for further exploration. This study significantly deepens our understanding of how cancer cells escape immune detection, highlighting a previously unknown form of immune evasion. The idea that cancer can directly impact the energy function of T-cells through mitochondrial manipulation adds a new dimension to the complex interplay between tumor cells and the immune system.
By identifying a potential intervention (inhibiting mitochondrial transfer), the study also paves the way for future research to develop therapies aimed at addressing these immune barriers. These findings may lead to the development of new drug strategies that combine mitochondrial transfer inhibition with other established therapies, potentially increasing the effectiveness of current treatments for a broader range of patients.
Conclusion
Cancer’s ability to evade the immune system is one of its most formidable characteristics. The discovery that cancer cells can hijack the immune cells by transferring defective mitochondria reveals a sophisticated and surprising method of immune evasion. This discovery not only explains why some immune-based treatments are ineffective but also provides a new potential strategy for enhancing cancer immunotherapy.
By targeting the mitochondrial transfer process, scientists have identified a possible path forward in tackling this aspect of cancer’s defense mechanism. This could lead to more successful treatments for patients who do not respond to traditional immunotherapy options, offering new hope in the fight against cancer. The discovery represents a significant step toward more effective, personalized cancer treatments, with the possibility of improving patient outcomes by overcoming one of cancer’s most insidious tactics.
References: Hideki Ikeda et al, Immune evasion through mitochondrial transfer in the tumour microenvironment, Nature (2025). DOI: 10.1038/s41586-024-08439-0
Jonathan R. Brestoff, Mitochondrial swap from cancer to immune cells thwarts anti-tumour defences, Nature (2025). DOI: 10.1038/d41586-025-00077-4