The search for answers to Alzheimer’s disease and other neurodegenerative disorders remains one of the most significant challenges in brain research. Alzheimer’s disease, in particular, has confounded scientists for decades due to its complex nature and the mysteries surrounding its onset and progression. Despite extensive research, the mechanisms behind its neurodegenerative effects—particularly the role of cholesterol and other lipids in the brain—are still not fully understood. Maciej J. Stawikowski, Ph.D., an assistant professor of chemistry and biochemistry at Florida Atlantic University’s Charles E. Schmidt College of Science, is leading the charge to investigate these phenomena.
Stawikowski, a member of the FAU Stiles-Nicholson Brain Institute, believes that cholesterol and other lipids may play a crucial role in how Alzheimer’s disease develops. His research points to the idea that understanding how these lipids move through the cells and interact could be key to uncovering the mechanisms behind Alzheimer’s disease and other neurodegenerative conditions.
“It’s well known that lipids and Alzheimer’s are linked,” said Stawikowski. “Lipid imbalance may lead to amyloid plaque formation—oversized protein clumps that disrupt cell function, which is a hallmark of Alzheimer’s.”
While the idea of lipids and their involvement in diseases like Alzheimer’s isn’t new, Stawikowski and his team have focused on a critical facet of this research: the movement and behavior of cholesterol within the cellular environment. Cholesterol plays a vital role in cellular function, particularly in the formation of cellular membranes, hormone production, stability, and signaling. However, when cholesterol movement becomes disrupted—particularly the way it travels between various cellular compartments—it may contribute to the development of neurodegenerative diseases, including Alzheimer’s.
Innovations in Fluorescent Cholesterol Probes
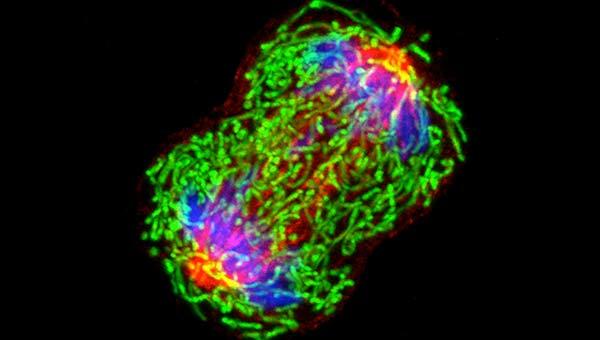
To better understand the relationship between lipids, cellular function, and Alzheimer’s, Stawikowski and his team have developed novel tools to track cholesterol in live cells. In particular, they have created innovative fluorescent cholesterol probes, known as Cholesteryl Naphthalimide Derivatives (CNDs). These probes were designed specifically to visualize and study cholesterol within living cells, offering unprecedented detail regarding the behavior and distribution of this essential lipid. The study, which was published in the journal Scientific Reports, highlights how these advanced tools are providing new insight into how cholesterol affects cellular processes.
“We have developed these cholesterol probes to give us a clearer picture of how cholesterol behaves within a cell and its role in various biological functions,” Stawikowski explained. “With these probes, we can now visualize cholesterol movement and distribution in live cells with unprecedented detail.”
Cholesterol is a fundamental component of the cellular membranes, and disturbances in its distribution or function can contribute significantly to diseases, including Alzheimer’s. Previous research has already established a connection between lipid imbalances and amyloid plaque formation, a pathological feature of Alzheimer’s disease. By uncovering how cholesterol behaves at a molecular level in the context of these plaques, Stawikowski and his team hope to build a clearer picture of how imbalances could lead to disease progression and offer pathways toward potential treatments.
Understanding the Probe Design and Its Mechanism
The novel cholesterol probes developed by the team are based on a 1,8-naphthalimide (ND) scaffold. This unique structure is well-known for its fluorescence properties, including large Stokes shifts and sensitivity to environmental changes. These properties make the probes particularly valuable in live-cell imaging, where changes in cellular environments—such as fluctuations in pH or the interaction with specific lipids—can be tracked and analyzed. By using the 1,8-naphthalimide scaffold, researchers can visualize cholesterol with a level of precision that previous cholesterol probes were unable to achieve.
These probes are not one-size-fits-all; rather, they can be customized for different experimental needs, enhancing their applicability in various research scenarios. By modifying their design—altering the head groups or linkers that are attached to the naphthalimide scaffold—scientists can tailor the probes to have specific properties, such as increased sensitivity or enhanced ability to target specific cellular structures.
The team identified several types of probes, each with distinct characteristics:
- Neutral Probes: These probes tend to aggregate easily but show limited ability to penetrate the cells. While their interactions within the cell are of interest, their use is more suited to studies involving larger-scale aggregates or structures.
- Charged Probes: Charged probes offer better solubility in the cellular environment, allowing for improved interaction with cellular membranes. These probes show enhanced cellular uptake and are more efficient in tracing cholesterol in different parts of the cell.
- Hydroxyl-Functionalized Probes: Probes with hydroxyl groups increase the number of hydrogen bonds they can form with lipids, improving their interaction with the cell membranes. This makes them particularly effective for studying complex membrane behavior and interactions with cholesterol.
Some of the most innovative versions of these CND probes are also sensitive to pH, allowing researchers to track cholesterol’s movement across various organelles within the cell. This can provide valuable insight into how cholesterol is distributed in environments that vary in acidity, such as lysosomes and lipid droplets. Traditional cholesterol probes often struggle to track dynamic movements or respond to changes in the cellular environment as effectively as the new probes.
Bridging the Gap in Alzheimer’s Research
In the context of Alzheimer’s disease, these probes provide a valuable tool for understanding how cholesterol dynamics contribute to the formation of amyloid plaques. Amyloid plaques consist of abnormal clumps of protein that disrupt cellular function, an issue central to Alzheimer’s pathology. Imbalances in the lipids, particularly cholesterol, can lead to these aggregates, exacerbating the disease. Understanding how cholesterol contributes to amyloid plaque formation is crucial for developing targeted therapeutic interventions.
The ability to visualize cholesterol in living cells will also provide clarity on its involvement in cellular signaling and how this contributes to overall brain health. Cholesterol’s central role in the stability and function of cell membranes means that understanding its behavior may reveal key insights into how cellular communications break down in neurodegenerative diseases.
As Stawikowski pointed out, “Cholesterol is essential for brain function, but its dysregulation could be a key factor in disease progression. Our new tools provide a window into how cholesterol impacts cellular processes and may help identify therapeutic targets for conditions like Alzheimer’s.”
These novel fluorescent cholesterol probes have potential applications beyond Alzheimer’s research. In the broader context of neurodegenerative diseases and lipid-related disorders, understanding how lipids like cholesterol move, interact, and regulate cellular processes is essential. Disruptions in these lipid processes could be common culprits in a range of diseases, including Parkinson’s and Huntington’s disease, making this tool particularly valuable for a wide range of scientific inquiries.
Further Applications and Future Directions
The potential applications for these probes extend far beyond Alzheimer’s disease. Stawikowski’s team believes that these tools could be used to explore lipid dynamics more broadly, including in studies of membrane biology, drug delivery systems, and other lipid-driven processes. Cholesterol’s role in cellular membranes is integral not just in the brain, but in nearly every cell of the body. The ability to study these dynamics with such high sensitivity and precision will contribute to new discoveries in the molecular mechanisms of many diseases.
One of the more exciting aspects of this research is the team’s integration of computer simulations with live-cell imaging. This dual approach is paving the way for new experimental techniques that enhance the utility of the CND probes. By using simulations alongside experimental imaging, researchers can predict how the cholesterol probes will behave under different cellular conditions and experiment with new ways to visualize cellular lipid behavior in real-time.
The versatility and adaptability of these cholesterol probes mark a significant leap forward in lipid-related research. As researchers continue to fine-tune their understanding of cholesterol’s role in both health and disease, these probes will undoubtedly play an essential role in future studies.
Conclusion: A Step Toward Targeting Lipids in Neurodegenerative Diseases
The groundbreaking work being done at Florida Atlantic University to track cholesterol in live cells has opened new possibilities for understanding how this lipid—and others—contribute to Alzheimer’s and other neurodegenerative diseases. By providing a clearer window into the mechanisms of lipid imbalance, these probes could help scientists identify new therapeutic targets and lead to the development of better treatments or preventive measures for Alzheimer’s disease.
Stawikowski and his team have pioneered a technology that stands to reshape how researchers approach Alzheimer’s disease and other disorders. The ability to visualize the movement of cholesterol within cells could unravel crucial details about cellular function and pave the way for novel drug therapies, giving hope to millions of individuals affected by these devastating diseases.
Reference: Vicente Rubio et al, Development and characterization of fluorescent cholesteryl probes with enhanced solvatochromic and pH-sensitive properties for live-cell imaging, Scientific Reports (2024). DOI: 10.1038/s41598-024-80958-2