Glass, although commonplace and seemingly simple, holds a unique place in materials science, particularly due to the unusual physics underlying its behavior. This material, often associated with transparency and fragility, has intrigued scientists for centuries because its molecular structure defies the typical patterns observed in either solid or liquid states. In fact, glass is a classic example of a material that doesn’t neatly fit into conventional categories but exists in an intermediate state between a liquid and solid.
Most materials, when they cool from a liquid to a solid, arrange themselves into orderly patterns known as lattices, which provide structure and rigidity. This ordering, however, does not happen in glass. Instead, glass solidifies without forming an orderly lattice, which gives it its defining properties. The molecules inside glass are arranged in a disordered state—reminiscent of liquids—but these molecules don’t have the freedom to move. While a glass may appear solid, its molecules are locked in a perpetually disordered and “frozen” state. Even though this may make the material rigid, its structure is inherently unstable, remaining out of equilibrium at low temperatures. This frozen disorder is one of the central mysteries that make the study of glass so compelling for scientists.
An even more puzzling phenomenon occurs when we look at supercooled liquids—another class of materials that occupy this same “in-between” region of matter. Unlike conventional liquids, where the fundamental particles can move freely, supercooled liquids remain in a liquid state despite being colder than their usual freezing point. Their particles fail to adopt the expected ordered structure of a solid, but similarly lack the freedom of movement associated with regular liquids. While supercooled liquids appear to be solids, their atomic behavior remains irregular, yet they retain the fluidity of a liquid. This strange and delicate balance between a solid and liquid phase is still not fully understood, and much more research is needed to decipher the complex physics involved in these states.
A recent study published in Nature Materials is shedding new light on these fascinating substances. Researchers at the Institute of Industrial Science, University of Tokyo, have employed advanced computer simulations to understand the behavior of fundamental particles in glassy supercooled liquids. This computational approach, which models how individual particles behave in a disordered system, has allowed them to probe deeper into the microscopic world of these enigmatic materials. Through this study, the scientists hope to uncover some of the hidden dynamics that influence how these materials behave when they are neither truly liquid nor fully solid.
One of the central ideas the researchers explored comes from the concept of the Arrhenius activation energy—a well-known theory in physics that describes the energy barrier a system must overcome for a particular process to take place. In conventional systems, this energy barrier is typically constant, and the rate of reaction follows what is called “Arrhenius behavior,” meaning the speed of a process exponentially decreases as the energy barrier increases.
However, in materials such as glassy liquids, things are much more complex. The arrangement of particles is irregular and often requires more than a simple random fluctuation to be rearranged. At low temperatures, the energy available to cause these fluctuations decreases, and in certain scenarios, particles must move cooperatively to maintain the material’s structural stability. The phenomena that arise in these cases are known as super-Arrhenius behaviors. This type of behavior deviates from typical expectations by suggesting that the process requires a large degree of cooperative rearrangements between particles, leading to unexpected dynamic patterns. Understanding how these unusual behaviors emerge is one of the major goals of ongoing research into glasses and supercooled liquids.
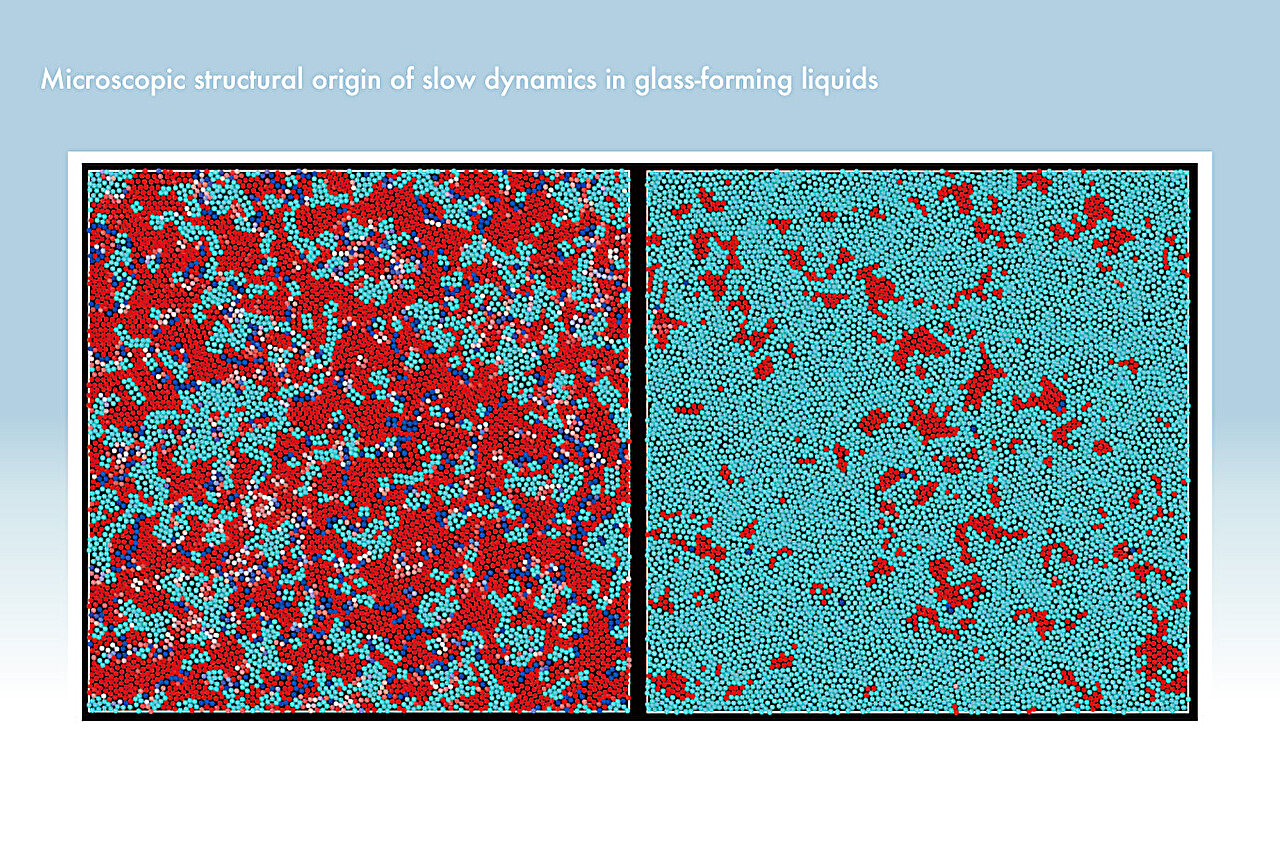
The Japanese team’s breakthrough came when they used computer modeling to simulate glass-forming liquids at the molecular level. Their study sought to uncover how the microscopic behaviors of individual particles could explain the observed changes in the material’s overall structural order and dynamic behavior. By closely examining the interactions at this scale, the researchers were able to demonstrate how these microscopic movements of particles could cause large-scale physical changes in the material, influencing its structural and dynamic properties.
The study’s lead author, Seiichiro Ishino, explains that the team used a computational approach to simulate and track these rearrangements in a model of glass-forming liquids. The results revealed the importance of a particular process called T1, which refers to an individual particle’s ability to rearrange in such a way that it either maintains or disrupts the local structural order within the material. In their findings, the team determined that if the T1 process is cooperative, allowing particles to reorganize in an orderly way, the overall structure of the liquid remains stable and predictable. In such cases, they observed what is called super-Arrhenius behavior—a phenomenon that drastically diverges from normal expectations based on conventional models of material behavior.
Alternatively, if the T1 process results in a disruption of this order, the particles move more independently and less predictably, following what is traditionally recognized as Arrhenius-like behavior. This insight helps further explain the unique characteristics of glassy liquids, as the varying behaviors of particle movement are intimately tied to the larger dynamic properties of the system. Importantly, their work provides the first direct connection between these fundamental particle interactions and the material’s observable properties.
What is even more intriguing is that this discovery could have broad implications not just for fundamental physics, but for technological applications as well. The researchers’ findings point to a new way of understanding how cooperative dynamics impact the formation and properties of glasses. These insights have the potential to revolutionize how we design and manufacture materials that depend on glass. Particularly in industries such as smartphone manufacturing, where glass is a key component, this research could lead to the development of stronger, more durable glass with specific desirable properties like increased resistance to shattering, better heat resistance, and a longer lifespan.
Hajime Tanaka, the senior author of the study, highlights the importance of these findings by emphasizing how this new perspective might lead to innovations in material design and glass manufacturing. “We expect our research to help refine our understanding of material dynamics on the atomic scale, making it possible to design glass with enhanced performance for a range of applications,” he says. In addition to creating more resilient glasses for electronics, these insights could be valuable for advancing other applications such as food packaging, optical components, and various types of coatings.
Glass might seem like an everyday object that we take for granted, but studies like these show that even the most familiar materials still hold countless mysteries waiting to be unraveled. Researchers continue to explore the complexities of glassy and supercooled systems, and this work represents a small but significant step toward understanding how to manipulate these materials at the atomic level, opening up new possibilities for technology and industry. This research offers not only a deeper understanding of glass but also paves the way for creating new materials with better control, greater performance, and enhanced durability—offering the potential for a wide range of advanced applications across multiple industries.
Reference: Microscopic structural origin of slow dynamics in glass-forming liquids, Nature Materials (2025). DOI: 10.1038/s41563-024-02068-8