Obesity is a global epidemic that affects over a billion people worldwide, contributing to various chronic diseases like cardiovascular conditions, Type 2 diabetes, sleep apnea, osteoarthritis, and certain types of cancer. For years, researchers have been investigating various therapies and treatments to help people battle obesity and its associated health risks. In the quest for a more effective solution, researchers from UT Southwestern Medical Center have discovered a promising new approach—targeting a receptor found in fat cells to induce weight loss. This receptor, known as the glucose-dependent insulinotropic polypeptide receptor (GIPR), plays a potentially pivotal role in therapies designed to combat obesity, highlighting its significance beyond previous understanding.
The research study, published in Cell Metabolism, demonstrates that genetically altering the fat cells of obese mice to produce more GIPR led to a remarkable reduction in body weight—more than a third of their original mass in just two weeks. This discovery presents GIPR not only as an influential protein in metabolic pathways but also as a potential star player in the development of weight loss treatments. The researchers believe that their findings could pave the way for therapies aimed directly at this receptor, opening up new possibilities for more effective obesity treatments.
Christine M. Kusminski, Ph.D., the study leader and an associate professor of Internal Medicine at UT Southwestern, emphasized the implications of these findings in terms of developing future weight loss interventions. Kusminski stated, “Our study brings GIPR in fat cells to light as a meaningful target for the development of future therapeutic interventions for the treatment of obesity and its associated metabolic diseases.”
The World Health Organization identifies obesity as a growing crisis, linked to a multitude of serious health conditions that heavily burden individuals and healthcare systems. Traditional weight loss drugs, many of which have been focused on the glucagon-like peptide-1 receptor (GLP-1R), have been used with relative success in reducing body weight. These drugs operate by targeting central brain areas that regulate hunger and satiety, successfully curbing appetite. Recently, however, a new class of weight-loss drugs has emerged that aims to interact with both GLP-1R and GIPR simultaneously. While initial clinical trials have shown that such drugs are particularly effective for those with obesity and Type 2 diabetes, the precise role of GIPR in enhancing the effects of GLP-1R targeting agents is still not fully understood.
To explore the role of GIPR, Dr. Kusminski and her team engineered mice with fat cells that produced an increased amount of GIPR. This provided a unique opportunity to explore the receptor’s activity in fat cells specifically. The team was led by Xinxin Yu, M.D., a research scientist at UT Southwestern, and Philipp Scherer, Ph.D., Professor of Internal Medicine and Cell Biology at the institution. By genetically increasing the expression of GIPR in fat cells, they observed a surprising and dramatic result. Mice that had been made obese experienced a stunning weight reduction of about 35% within two weeks after the overproduction of the GIPR protein in their fat cells.
Additionally, when the researchers overexpressed GIPR in fat cells of normal-weight mice, these animals were protected from obesity despite being fed a high-fat diet. The findings suggested that targeting this receptor in fat cells could serve as a preventive or therapeutic measure against obesity.
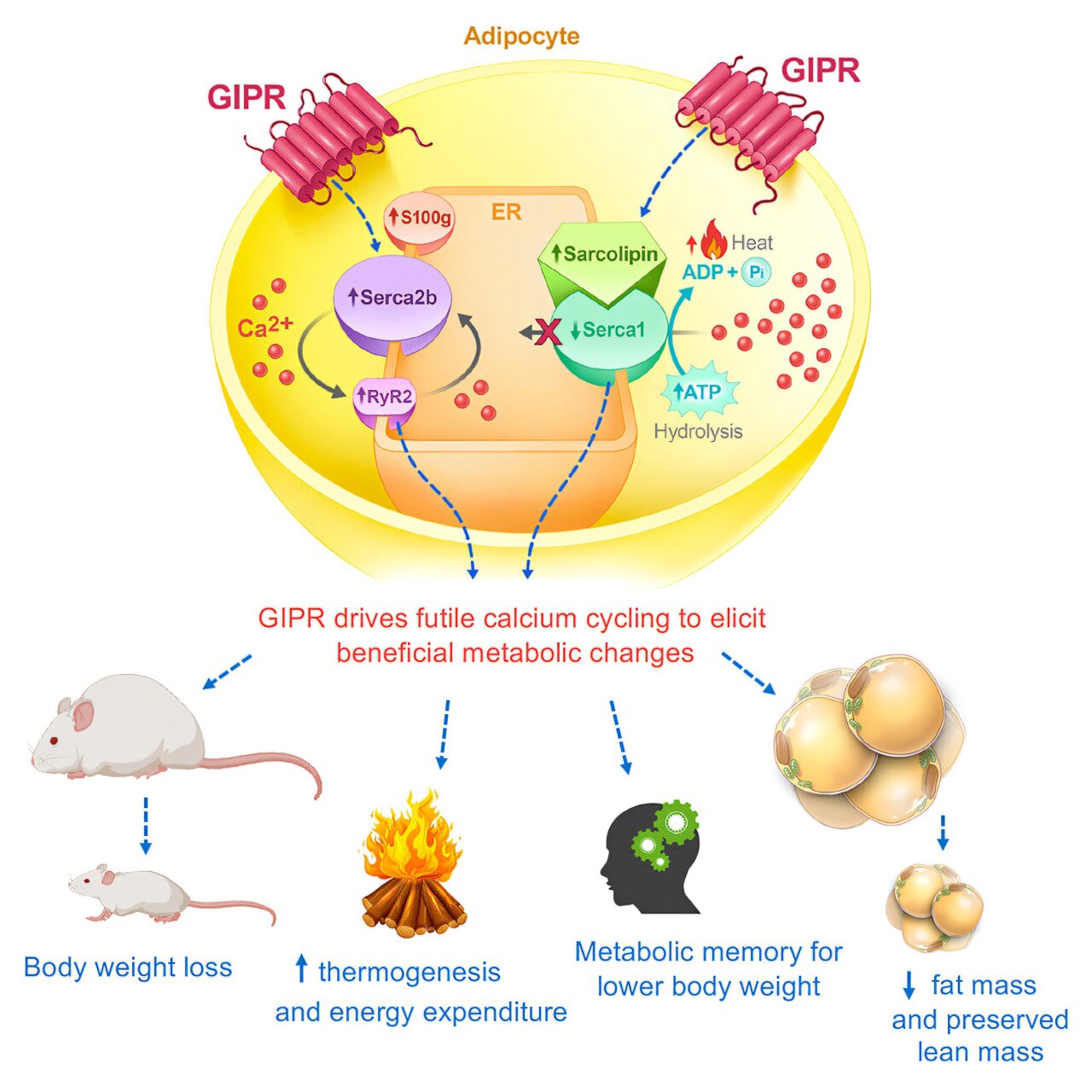
Intrigued by this significant weight loss, the team next sought to identify the molecular pathways behind the extraordinary energy-burning effects of GIPR activation. Upon further investigation into the animals’ metabolic systems, they identified changes in key gene pathways that were linked to increased cellular energy expenditure. More specifically, they found that the overproduction of GIPR in fat cells resulted in increased activity of the sarco/endoplasmic reticulum calcium ATPase (SERCA) pathway, a process responsible for moving calcium within cells. The increased activity of SERCA involves using energy to transport calcium ions, and in this case, energy was being consumed without effective calcium transport taking place. This was determined to be an inefficient but energy-consuming process, leading to excessive energy expenditure. As a result, mice overproducing GIPR in their fat cells burned more ATP—a molecule critical to cellular functions—without gaining any benefits in terms of calcium transport. The futile calcium cycling contributed to a higher energy burn, thus resulting in weight loss.
In a surprising turn of events, the researchers found that when they stopped the overproduction of GIPR after several weeks, the obese mice did not regain the weight they had lost. They even seemed to display what has been described as “metabolic memory,” a protective effect against obesity, which prevented the rapid return of fat mass despite the cessation of the genetic alteration. This stands in contrast to the typical response seen in human patients who stop taking existing weight-loss medications—where rapid weight gain usually follows the discontinuation of treatment.
Dr. Kusminski highlighted that understanding the ways in which GIPR works in fat cells offers crucial insights into why therapies targeting both GIPR and GLP-1R can lead to more significant weight loss than therapies targeting GLP-1R alone. The current research paves the way for considering GIPR as a key player in the quest for weight loss solutions. She added, “Having a better understanding of how GIPR operates in fat cells helps to explain why targeting GIPR, in addition to GLP-1R, causes individuals with obesity to lose more weight than those who take GLP-1R drugs.”
Furthermore, Kusminski emphasized the potential of using GIPR-specific drugs—or those that act on GIPR as part of a combined approach—to generate substantial benefits in the fight against obesity. A broader exploration of GIPR-based weight loss therapies might lead to innovative options that move beyond current treatments, ultimately helping individuals struggling with obesity improve their health and quality of life.
Obesity is not just a cosmetic issue—it is a severe metabolic condition with broad health implications. Therefore, finding new, effective treatment options remains crucial. The UT Southwestern Medical Center team’s breakthrough work suggests that GIPR could represent a novel target in the fight against obesity, offering both an alternative and complementary approach to existing weight loss therapies.
As the search for effective solutions continues, these findings may lay the groundwork for therapies that tackle not just the symptoms of obesity but its root causes at the cellular level. The promise of using GIPR to manipulate metabolic pathways offers hope for more sustainable treatments, giving researchers and patients alike new tools in the ongoing battle against this challenging global health issue. By harnessing the potential of this relatively overlooked protein, it’s possible that science could soon bring effective, long-lasting weight loss solutions to people who need them most.
Reference: Xinxin Yu et al, The GIP receptor activates futile calcium cycling in white adipose tissue to increase energy expenditure and drive weight loss in mice, Cell Metabolism (2024). DOI: 10.1016/j.cmet.2024.11.003
Think this is important? Spread the knowledge! Share now.