In the field of chemistry, there is an increasing push towards more sustainable practices that minimize the negative environmental impact of traditional industrial processes. Mechanochemistry stands out as a particularly innovative approach in organic synthesis because, unlike conventional methods, it does not rely on solvents. Solvents have long been used in organic reactions to dissolve reactants and facilitate chemical transformations. However, solvents often end up as waste products, causing significant environmental concerns and waste disposal issues. Mechanochemistry offers an alternative that completely eliminates the need for solvents, making it an environmentally friendly technique with great promise for sustainable chemical production.
In mechanochemical reactions, the reactants typically remain in solid form, a sharp contrast to conventional organic synthesis methods, where liquids are commonplace. The key advantage of this solid-state synthesis lies in the unique conditions created when mechanical forces, such as those generated by grinding, milling, or other mechanical agitation, are applied. This results in chemical reactions occurring directly at the interface between solid reactants. Notably, mechanochemistry also makes it possible to carry out reactions involving materials that are poorly soluble in traditional solvents.
The Mysterious Forces Behind Mechanochemical Reactions
While the benefits of mechanochemistry are clear, there remain many mysteries surrounding the mechanisms that govern these reactions. It is widely acknowledged that applying mechanical forces accelerates the reaction process, but the precise nature of these forces and their influence on the molecular scale have not been fully understood. The applied force in mechanochemical processes is typically introduced by a device like a ball mill, where high-speed collisions of hard materials cause friction and compression in the reaction vessel. These mechanical forces break down the solid materials, increasing the contact between reactants and generating sufficient energy to trigger reactions.
Despite the increased use of mechanochemistry, the field’s theoretical foundation remains in its early stages when compared to traditional organic chemistry, where extensive theoretical models have been developed over centuries. In mechanochemistry, many essential aspects such as reaction kinetics—how quickly reactions occur in the presence of mechanical forces—are not yet fully explained. This gap in understanding must be addressed before mechanochemistry can become a conventional strategy for industrial organic synthesis, which would lead to broader applications and increased adoption.
New Theoretical Framework for Mechanochemical Reactions
A significant step forward in understanding the influence of mechanical forces on organic reactions has been made by a team of researchers led by Associate Professor Tetsuya Yamamoto at the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Japan. Their pioneering study, published in the journal RSC Mechanochemistry, aims to fill this knowledge gap by providing a theoretical framework for predicting the reaction rates in mechanochemical reactions. Their work was the result of an exceptional collaboration between experts specializing in organic chemistry and rheology, a branch of physics focused on the flow and deformation of matter.
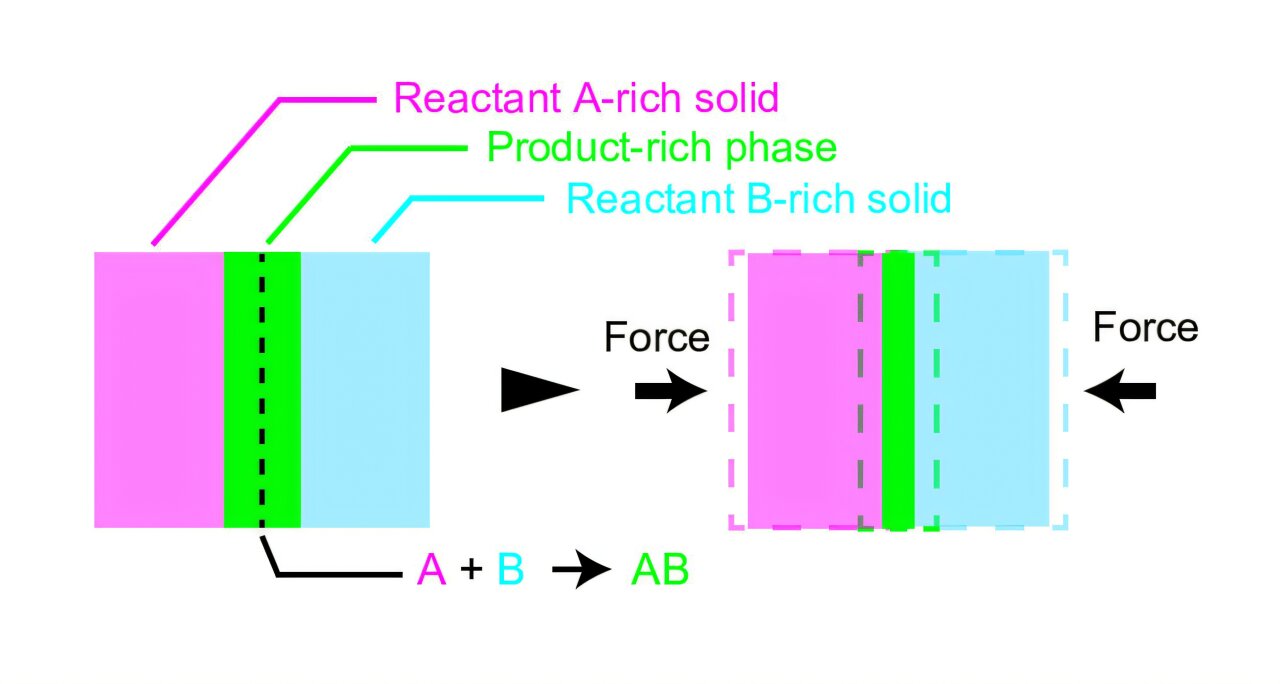
Through their groundbreaking research, the team developed a new theory focused on the interface between solid reactants, a central aspect of mechanochemical reactions. In a typical mechanochemical process, chemical reactions occur between particles at their contact surfaces, also known as interfaces. The interactions between these particles can be dramatically affected by the mechanical forces applied by the ball mill or other mechanical devices.
The team’s new theory posits that the layer formed at the interface—comprising mainly the products of the chemical reaction—plays a crucial role in determining the reaction rate. As reactants undergo the grinding or milling process, a product layer forms at the contact points where the reaction is occurring. In this new framework, the formation of this product layer has a direct impact on the overall speed at which the reaction takes place.
How Mechanical Forces Accelerate Reactions
One of the major insights from the study is the mechanism by which mechanical forces accelerate chemical reactions. When the ball in a ball mill collides with the solid reactants, force is applied directly to the interfaces where chemical interactions occur. This force causes the product-rich layer at the interface to thin, which in turn results in more rapid collisions between the reactant particles. These more frequent collisions increase the rate at which reactants are converted into products, leading to a faster overall reaction.
The results of this research suggest that the acceleration of the reaction is not just due to the increase in the temperature or energy from the mechanical force, but rather due to its direct influence on the molecular-scale interactions between the reactants. This gives us important insight into how grinding and milling do more than just agitate a reaction; the mechanical stress actually optimizes the conditions at the molecular level, leading to better and faster reactions in comparison to traditional solution-based syntheses.
Advancing the Understanding of Reaction Kinetics in Mechanochemistry
The theoretical framework developed by Yamamoto and his team is notable in that it is the first attempt to construct a kinetic theory for mechanochemical reactions that incorporates the role of interfaces between solid reactants. By applying this model, researchers can gain a deeper understanding of how mechanical forces contribute to reaction acceleration and what parameters need to be optimized to make mechanochemical reactions more efficient and predictable. With this kind of detailed understanding, the possibility arises for systematic optimization of mechanochemical processes, which could revolutionize how chemical reactions are conducted in both academic and industrial settings.
However, this is only the beginning, and as highlighted by Associate Professor Koji Kubota, the second author of the study, the detailed mechanisms of mechanochemical processes are still largely unknown. Although their theoretical framework is a promising start, further refinement and experimental validation will be necessary to fully unveil the complexities of these reactions. The hope is that with continued interdisciplinary collaboration between organic chemists, rheologists, and other specialists, this research will lay the foundation for more robust, generalized models that can help predict and control the outcomes of mechanochemical reactions across various chemical systems.
Implications for Industrial Applications
If mechanochemical reactions become better understood and more widely applicable, their potential to impact industrial processes could be enormous. Industries that rely heavily on chemical synthesis, such as pharmaceuticals, material science, and renewable energy production, could benefit significantly from this solvent-free technique. By eliminating the need for solvents, mechanochemistry not only reduces the environmental impact of chemical processes but also opens up new avenues for creating materials and products that might otherwise be challenging to produce using traditional methods. Furthermore, mechanochemistry could lower the energy consumption and costs of chemical synthesis, making it a valuable tool for more sustainable industrial practices.
Conclusion
Mechanochemistry holds tremendous promise for transforming the field of organic synthesis and chemical reaction design. The recent theoretical advancements made by Tetsuya Yamamoto and his collaborators represent a critical step towards understanding how mechanical forces influence chemical reactions at the molecular level. As researchers continue to refine and expand on this work, mechanochemistry has the potential to revolutionize industrial chemical processes, enabling sustainable production and efficient synthesis of materials, all while contributing to a more environmentally responsible future.
In sum, as the understanding of mechanochemical reactions continues to deepen, there is no doubt that these solvent-free methods will play an increasingly central role in future scientific research and industrial applications, paving the way for innovative solutions to some of the most pressing challenges in chemistry and sustainability.
Reference: Tetsuya Yamamoto et al, Scaling theory for the kinetics of mechanochemical reactions with convective flow, RSC Mechanochemistry (2024). DOI: 10.1039/D4MR00091A