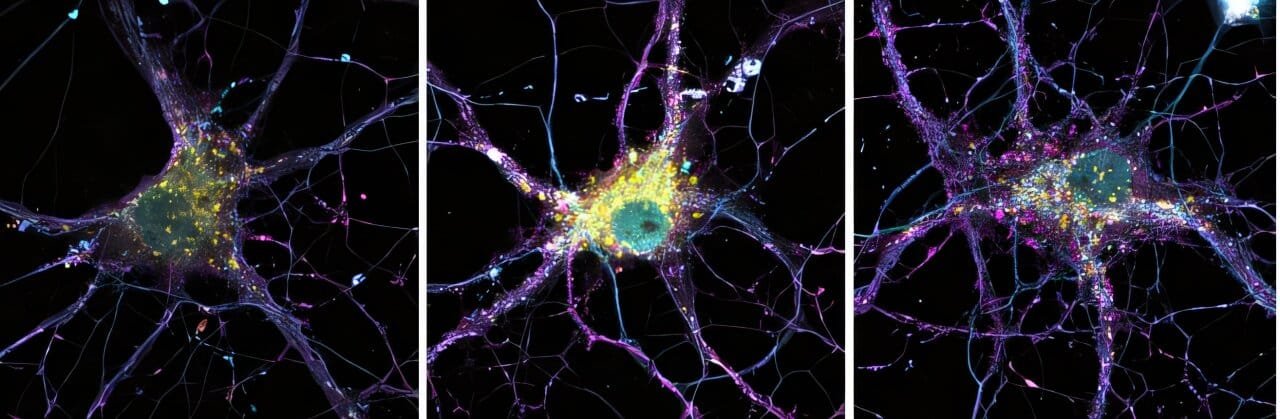
Alzheimer’s disease, a devastating neurodegenerative disorder, progressively erodes memory, cognitive functions, and overall mental faculties. Affecting millions globally, it remains one of the most challenging conditions in neuroscience and medicine. While most cases of Alzheimer’s disease are sporadic and occur in older populations, familial Alzheimer’s disease (FAD) is a rare inherited form of the disorder. It emerges at an earlier age, often in individuals as young as their 30s or 40s, and is linked to mutations in specific genes, notably APP (amyloid precursor protein), PSEN1 (presenilin-1), and PSEN2 (presenilin-2).
The role of PSEN2 mutations in FAD has historically been less understood compared to its counterpart PSEN1. However, groundbreaking research led by Professor Wim Annaert at the VIB-KU Leuven Center for Brain & Disease Research has recently illuminated the complex role of PSEN2 mutations in advancing the pathology of Alzheimer’s disease. Their findings, published in Nature Communications, reveal how alterations in PSEN2 disrupt crucial cellular processes and exacerbate the disease’s progression. This discovery not only deepens our understanding of FAD but also opens potential avenues for therapeutic interventions.
Alzheimer’s disease is hallmarked by the accumulation of amyloid plaques in the brain—dense clumps of β-amyloid (Aβ) peptides that form through the processing of APP by the γ-secretase complex. This enzyme complex is essential for cutting APP into smaller fragments, some of which, including Aβ, can aggregate to form toxic plaques. The γ-secretase complex is comprised of different variants, with either PSEN1 or PSEN2 as a critical component. While both variants perform similar roles, their precise locations within the cell and their functional nuances differ, hinting at unique contributions to cellular health and disease mechanisms.
The study by Professor Annaert’s team investigated how mutated PSEN2 influences the progression of Alzheimer’s by focusing on its role in critical cellular systems. Using advanced mouse models that replicate aspects of FAD, the researchers compared the outcomes of losing PSEN2 entirely to the effects of carrying a mutated form of the gene. Both scenarios revealed accelerated amyloid plaque formation and severe memory impairments, shedding light on how PSEN2 dysfunction disrupts normal brain activity.
Memory and cognitive deficits in Alzheimer’s are strongly linked to the deterioration of the hippocampus—a brain region critical for forming and retrieving memories. In the mouse models, disruptions to PSEN2, either through deletion or mutation, resulted in significant structural and functional damage to the hippocampus. Synaptic communication, the process by which neurons relay signals to one another, was also impaired. One of the critical features of healthy synapses is long-term potentiation, the ability of these connections to strengthen with use, which underpins learning and memory. However, in the absence or malfunctioning of PSEN2, this strengthening process was compromised, further hindering cognitive capabilities.
A deeper examination of neuronal health revealed that PSEN2 mutations affect endosomes and lysosomes—vital cellular organelles responsible for recycling and breaking down proteins. Endosomes act as sorting hubs for cellular materials, directing them to their appropriate destinations, while lysosomes serve as the “garbage disposal” system of the cell, degrading and recycling unusable components. The research showed that PSEN2 plays an essential role in maintaining the balance of these systems, particularly in neurons. Mutations in PSEN2 disrupted these processes, leading to the accumulation of toxic APP fragments, including Aβ peptides. These fragments not only contribute to plaque formation but also create bottlenecks in the degradation and recycling of other cellular components.
The resulting cellular dysfunction has cascading effects. When lysosomes and endosomes become overwhelmed or impaired, neurons struggle to maintain proper synaptic function and clear waste products. This amplifies the damage caused by amyloid plaques and accelerates synaptic and cognitive decline. As Anika Perdok, the first author of the study, stated, “PSEN2 mutations exert a double-edged impact—on one hand, promoting amyloid toxicity, and on the other, undermining basic cellular maintenance, further driving brain degeneration.”
These findings offer significant implications for Alzheimer’s research. They emphasize that beyond the visible effects of amyloid plaque buildup, the underlying cellular dysfunction caused by PSEN2 mutations represents a critical target for intervention. Addressing these cellular pathways may not only help mitigate the effects of amyloid toxicity but also preserve neuronal function and slow disease progression.
Professor Annaert underscored the potential therapeutic implications of their work, suggesting that restoring the normal functioning of endosomes and lysosomes, or modulating the activity of the γ-secretase complex within these organelles, could form the basis of new treatments for FAD. While translating these insights into clinical therapies will require extensive further research, the study provides a promising foundation for developing targeted interventions.
This research also highlights the need to understand the diverse mechanisms underlying different forms of Alzheimer’s. Although familial Alzheimer’s disease represents only a small fraction of cases, studying its genetic roots provides invaluable clues about the broader pathology of Alzheimer’s and related neurodegenerative disorders. The insights gained from FAD could lead to strategies that benefit patients with sporadic, late-onset Alzheimer’s, the most prevalent form of the disease.
Beyond therapeutic implications, this study reflects the importance of fundamental research in unraveling complex biological systems. By investigating how PSEN2 mutations disrupt cellular homeostasis, the researchers have painted a clearer picture of the multifaceted processes driving Alzheimer’s progression. Such discoveries not only enhance our scientific understanding but also fuel hope for addressing one of humanity’s most challenging health crises.
In the future, the focus on pathways influenced by PSEN2—including those regulating amyloid processing, lysosomal degradation, and synaptic health—could pave the way for precision medicine approaches in Alzheimer’s care. Early interventions targeting these pathways might delay disease onset or slow its progression, offering hope to families affected by this devastating condition. The findings by Professor Annaert and his team mark an important step in this direction, emphasizing the potential of targeted research to unravel the complexities of Alzheimer’s disease and bring new possibilities to light for its treatment.
Reference: Anika Perdok et al, Altered expression of Presenilin2 impacts endolysosomal homeostasis and synapse function in Alzheimer’s disease-relevant brain circuits, Nature Communications (2024). DOI: 10.1038/s41467-024-54777-y