Recent research, led by University of Toronto (U of T) researchers in collaboration with Insilico Medicine, has revealed an exciting possibility for revolutionizing the drug discovery process. By combining quantum computing, generative artificial intelligence (AI), and classical computing methods, the team has demonstrated a powerful new approach to designing molecules that target cancer-related proteins previously considered “undruggable.” This breakthrough, published in Nature Biotechnology, specifically focuses on tackling KRAS, a protein central to many cancer types, offering hope for the development of more effective treatments.
The Importance of KRAS in Cancer
KRAS is a protein that plays a critical role in controlling cell growth and differentiation. Mutations in the KRAS gene result in its constant activation, leading to uncontrollable cell division, a hallmark of cancer. These mutations are responsible for approximately 25% of all human cancers, including those affecting the lung, pancreas, and colon. Despite their prevalence, KRAS mutations have been notoriously difficult to target with drugs.
Currently, only two FDA-approved drugs specifically address mutant KRAS, but they have limited efficacy, often extending patient survival by just a few months compared to standard chemotherapy. This emphasizes the urgent need for the development of new and more effective therapies targeting mutated KRAS.
The research team led by Alán Aspuru-Guzik, a professor of chemistry and computer science at U of T, alongside collaborators at Insilico Medicine, has made significant strides in tackling this challenge by utilizing AI and quantum computing. According to Aspuru-Guzik, “It’s an exciting time to be working at the interface of chemistry, quantum computing, and AI,” underlining the groundbreaking potential of integrating these technologies to accelerate the drug discovery pipeline.
The Quantum-AI Drug Discovery Method
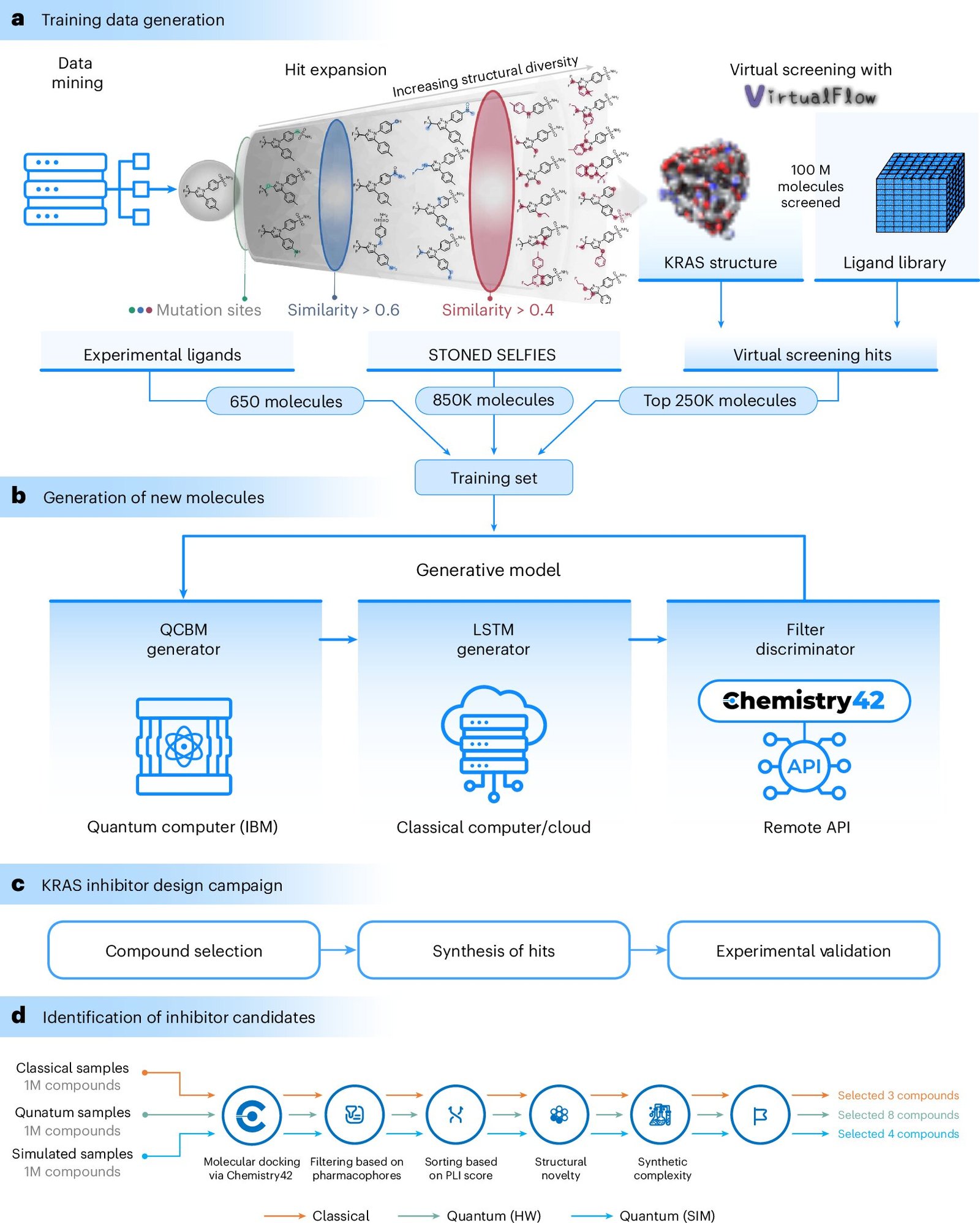
The study marks a first-of-its-kind application of quantum computing in combination with AI to find drug molecules targeting the KRAS protein. The approach represents a departure from traditional drug discovery methods, which generally involve screening large libraries of existing compounds to identify potential candidates.
In the study, the team combined quantum computing with classical computing techniques to design new drug molecules. Initially, the researchers trained their models using a unique dataset consisting of 1.1 million molecules. This dataset included 650 molecules experimentally proven to block KRAS and over 250,000 molecules sourced from VirtualFlow, an open-source platform for large-scale virtual screening.
Insilico Medicine’s Chemistry42 AI engine then played a crucial role by screening the designed molecules, evaluating their potential for binding to KRAS, and identifying the top 15 most promising candidates. These candidates were subsequently selected for lab testing. Among these, two molecules showed remarkable potential, effectively interacting with multiple versions of the KRAS protein in living cells. Their successful targeting of mutant KRAS indicates that these molecules could play a role in the development of new anti-cancer therapies.
Shortening the Drug Discovery Timeline
The study has far-reaching implications for drug discovery, particularly when it comes to reducing the extensive time required for early-stage research. Traditional drug discovery approaches are both costly and slow, often involving years of laboratory screening. By utilizing AI and quantum computing, the research team was able to more efficiently design and test new molecules.
Igor Stagljar, a professor of biochemistry and molecular genetics at U of T’s Donnelly Centre and co-investigator on the study, emphasizes the benefits: “Computational approaches like this have the potential to shorten the preclinical phase of drug discovery by years.” By employing these next-generation technologies, the team could not only speed up the discovery of new drug candidates but also alleviate the logistical complexities and high costs traditionally associated with the process.
For example, the quantum-enhanced methodology used in the study allows researchers to sidestep the physical constraints of screening and storing vast chemical libraries. The ability to conduct extensive simulations and tests using cloud-based systems frees researchers from maintaining large repositories of chemicals and expensive robotic systems for screening.
The Promise of Quantum Computing in Drug Discovery
Though the results of this study illustrate the potential of quantum computing to significantly advance drug discovery, it’s important to note that this research represents a proof-of-principle. Aspuru-Guzik cautioned, “While we show that a quantum computer can help with drug discovery, that doesn’t mean it is definitively better than classical computing for this task yet.”
The use of quantum computing in this context demonstrated a crucial step forward, suggesting that as quantum computing technologies mature, they can contribute even more effectively to pharmaceutical research. However, this study does not yet demonstrate that quantum computing provides a substantial advantage over classical computers in terms of molecular discovery. Rather, it provides early evidence that quantum computing can be seamlessly integrated into AI-driven, accelerated drug discovery pipelines.
“We hope that as quantum computers grow in power, our algorithms will perform better and better,” said Aspuru-Guzik, further underscoring the long-term potential of quantum computing for drug discovery.
Expanding to Other “Undruggable” Targets
Building on their success with KRAS, the research team is now applying their hybrid quantum-classical approach to other proteins previously thought to be “undruggable.” Proteins like KRAS are small in size and lack the distinctive surface features that make it easier for drugs to bind to them, presenting a significant obstacle in the design of effective treatments.
The team is working with a variety of proteins that share these characteristics and are implicated in cancers, neurodegenerative diseases, and other serious health conditions. Using the hybrid model, the researchers aim to unlock new opportunities to target these otherwise elusive proteins.
The collaboration between U of T and Insilico Medicine extends beyond KRAS, with the intention to tackle a broad spectrum of challenging proteins. The researchers are particularly focused on small proteins with surfaces that resist easy drug binding—an area that presents a significant hurdle for traditional drug design techniques.
Industry Collaboration for Accelerating Progress
The Accélération Consortium, a strategic initiative at the University of Toronto, has played a vital role in bringing together the research community, industry, and government stakeholders to foster collaborative advancements in AI-driven drug discovery. The consortium’s involvement in the project facilitated the partnership between U of T and Insilico Medicine, creating a unique synergy between academic and commercial entities.
Alex Zhavoronkov, founder and CEO of Insilico Medicine and a co-author of the study, points to the promise of such collaborations in driving progress in medicine: “As many as 85% of all human proteins are thought to be ‘undruggable,'” he notes, emphasizing the gravity of the challenge faced by researchers. “AI is uniquely positioned to help address this issue. The collaboration between U of T and Insilico Medicine is a great example of how the startup and university ecosystems can leverage our collective expertise to drive progress toward better health for all.”
Moving Toward Preclinical Testing
With the success of their hybrid quantum-classical approach, the researchers are continuing to refine their work. They aim to optimize their top KRAS candidates further and move these promising compounds into preclinical testing. If successful, these candidates could provide much-needed improvements over existing therapies and make a significant impact in the fight against cancer.
This project’s success offers a glimpse into a future where quantum computing and AI work together not only to accelerate the process of drug discovery but also to bring innovative therapies to the clinical stage more quickly and efficiently than ever before.
Conclusion
The joint efforts of the University of Toronto and Insilico Medicine have successfully demonstrated that quantum computing and AI are not just complementary to classical drug discovery methods but can form an integrated, powerful tool for overcoming longstanding barriers in the development of new medicines. Their work on KRAS—once considered an undruggable target—illustrates the potential to develop more effective cancer therapies and opens the door to tackling numerous other hard-to-target proteins.
As AI and quantum technologies continue to advance, they could significantly shorten the timeline of bringing new drugs to market, reduce costs, and, ultimately, help provide solutions to some of the most pressing healthcare challenges faced by patients worldwide. With further optimization and additional research, these innovative methods could pave the way for transformative breakthroughs in cancer therapy and beyond.
Reference: Mohammad Ghazi Vakili et al, Quantum-computing-enhanced algorithm unveils potential KRAS inhibitors, Nature Biotechnology (2025). DOI: 10.1038/s41587-024-02526-3
Loved this? Help us spread the word and support independent science! Share now.