Signals transmitted from the brain to motor neurons are crucial for the initiation and control of muscle movement. While the motor neurons directly control muscle contraction, the communication process is not as simple as a direct connection from the brain. Signals are often routed through a group of intermediary cells in the spinal cord known as interneurons before reaching motor neurons. This pathway plays a crucial role in shaping motor commands, but the exact way in which brain regions communicate with these diverse interneurons has been poorly understood for a long time.
The complexity of this system has presented challenges for researchers who aim to untangle the intricate connections between the brain and spinal cord. To tackle this problem, scientists at St. Jude Children’s Research Hospital took a groundbreaking step in creating a comprehensive whole-brain atlas. This tool, designed to visualize the regions of the brain that send direct inputs to V1 interneurons, a class of neurons essential for movement, has the potential to unravel this complex neural network. By providing a detailed three-dimensional interactive website, the research team created an important resource to further explore how the brain communicates with the spinal cord, with a focus on motor control.
According to Dr. Jay Bikoff, corresponding author of the study and an expert in the Department of Developmental Neurobiology at St. Jude, the motor system has long been understood as a distributed network, where motor neurons in the spinal cord are responsible for executing muscle movements. However, as Bikoff points out, motor neurons don’t function in isolation; rather, they are heavily influenced by diverse interneurons that modulate their activity. The brain’s control over these interneurons plays a pivotal role in regulating how motor neurons generate movements, but mapping out these circuits had remained a major challenge.
Interneurons, crucial intermediaries in this process, come in a vast array of forms, each with specific functional and molecular properties. Understanding how these neurons work together requires unraveling a maze of complex, interconnected cells, akin to untangling an extremely challenging and multi-dimensional puzzle. As co-first author Dr. Anand Kulkarni describes, understanding the entire system is particularly difficult since it involves over 3 billion years of evolutionary development—meaning that it’s not just a modern challenge but one that spans the deep history of life’s biological complexity.
In recent years, studies have confirmed that interneurons are not a homogenous group. Instead, these cells exist in many different subclasses, each playing a distinct role in the nervous system. Despite this growing body of knowledge, the specific relationships between these interneuron subclasses and their role in motor control remain largely undefined. A critical step in understanding the functional anatomy of motor control is understanding how the brain communicates with these distinct populations of interneurons and shapes their output.
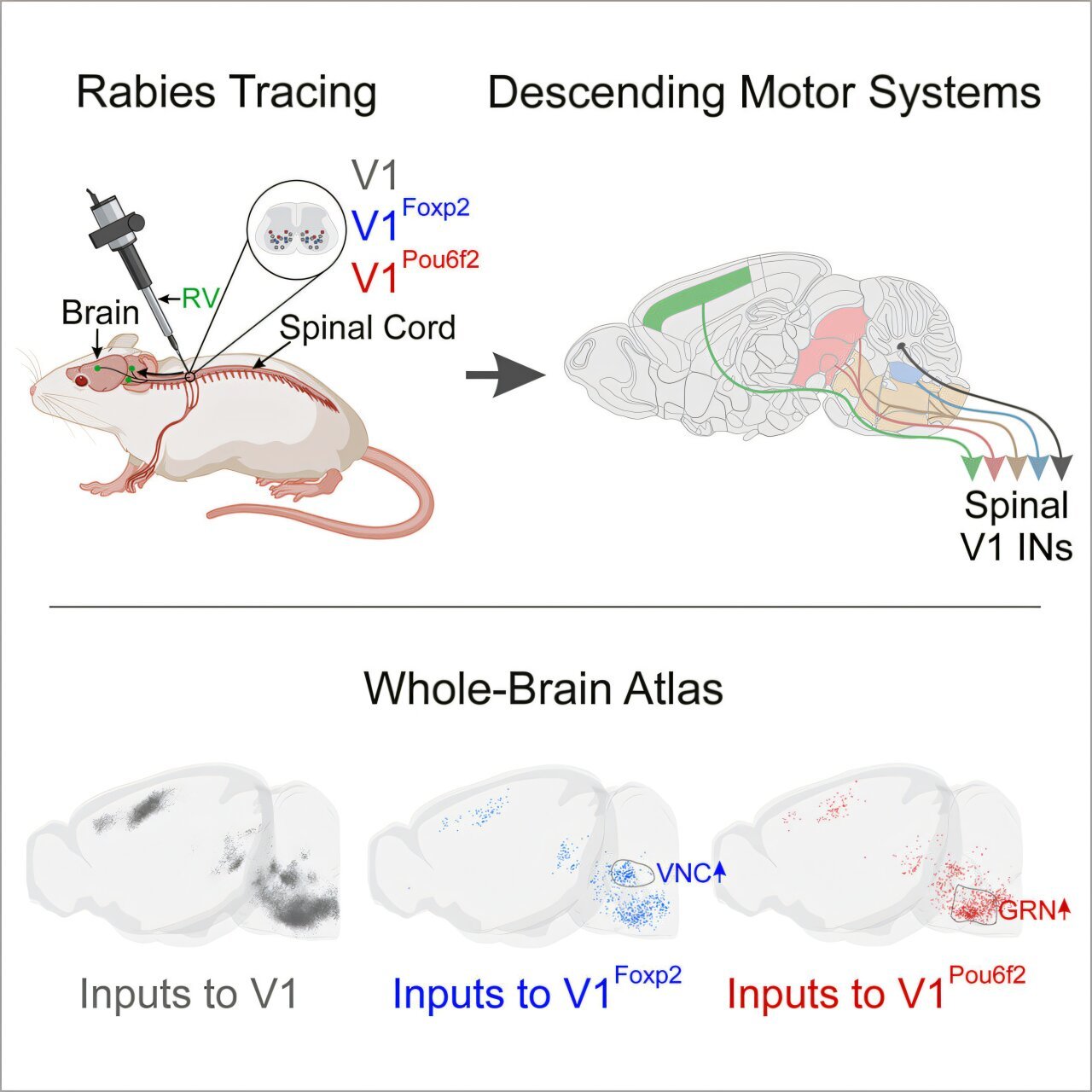
To explore these connections, the researchers at St. Jude used an innovative approach involving a genetically modified version of the rabies virus. While rabies is a devastating neurovirus, scientists can harness certain aspects of its biology to study neural connections. The key feature of the modified virus is its inability to spread from neuron to neuron. Without a key protein called the glycoprotein, the rabies virus cannot transfer between neurons as it typically would. This limitation allowed the researchers to manipulate the virus’s spread, ensuring that it remained within its population of origin, making it easier to trace its origin and path.
By carefully reintroducing the missing glycoprotein in specific populations of interneurons, they were able to encourage the virus to make a single hop between neurons, tracing its path to identify the regions of the brain connected to those particular interneurons. This methodology allowed the team to pinpoint brain regions directly sending signals to the V1 interneurons, a class known for their essential role in generating movement.
The work yielded a groundbreaking discovery: the connections from various regions of the brain to the V1 interneurons were more complex and widespread than previously realized. As Dr. Bikoff explains, the V1 interneurons themselves form a highly heterogeneous group. With a varied set of roles, it was crucial for the researchers to assess as many of these cells as possible to understand the breadth of influences these interneurons receive.
To document and visualize these findings, the researchers turned to a powerful technique called serial two-photon tomography. This advanced imaging technology involves scanning the brain in micron-thick slices, capturing detailed, fluorescently labeled neurons from multiple angles. The resulting three-dimensional images provide highly detailed depictions of the brain’s wiring. With this tool, the researchers generated an entire reference atlas, marking which regions of the brain communicate directly with the spinal interneurons and uncovering the far-reaching landscape of connections governing motor control.
In addition to mapping these neural circuits, the research team constructed an interactive 3D web atlas that is publicly accessible. This innovative resource offers a new way for researchers across the globe to explore the anatomical connections that play a role in motor control. The inclusion of interactive components allows anyone to navigate the brain’s connections to the spinal interneurons, facilitating new studies and fostering deeper exploration into how the brain controls movement.
This anatomical map is significant because, as Dr. Bikoff points out, it serves as more than a visual tool—it is a hypothesis-generating engine that will be invaluable for future research. By understanding the connections between the brain and specific interneurons involved in movement, scientists can make more informed predictions about how brain activity influences motor behavior. The research not only improves the fundamental understanding of the brain’s role in movement but also opens new avenues for exploring potential interventions in motor dysfunction.
Understanding how neural circuits influence movement will have major implications in several fields, particularly neuroscience and neurology. It can lead to new approaches in treating diseases like Parkinson’s disease, stroke, and other motor-related disorders. With motor control governed by so many factors at the level of both the brain and spinal cord, deciphering these interconnections is a major step toward developing better treatments and therapies for conditions that impair movement.
In addition to its application in medicine, this work contributes to a greater understanding of brain function as a whole. Decoding how different regions of the brain shape complex behaviors like motor movements—and how these behaviors emerge from the coordinated activity of neurons—has broader implications for how we study behavior and neurological processes.
Ultimately, this research paves the way for the next generation of neuroscientific inquiry, providing a solid foundation for scientists to build on. It highlights how fine-tuning the tools and methods available to researchers can enhance our knowledge of the complex world within the nervous system. As more data becomes available through interactive resources and advances in imaging technology, biologists and neuroscientists will gain new insights into how the brain coordinates movement at the molecular and cellular levels.
This new comprehensive whole-brain atlas is much more than a visual tool; it represents a breakthrough in the effort to decipher one of the brain’s most intricate functions: how the brain communicates with the spinal cord to enable movement. The significance of this work lies not only in its present contribution but in how it will catalyze the next wave of discoveries in neurobiology, health, and behavioral science. The ability to map and visualize these connections is the first step toward fully unraveling the complexities of the brain and spinal cord and will continue to fuel research into movement, behavior, and neurological health for years to come.
Reference: A brain-wide map of descending inputs onto spinal V1 interneurons, Neuron (2024). DOI: 10.1016/j.neuron.2024.11.019. www.cell.com/neuron/fulltext/S0896-6273(24)00876-6