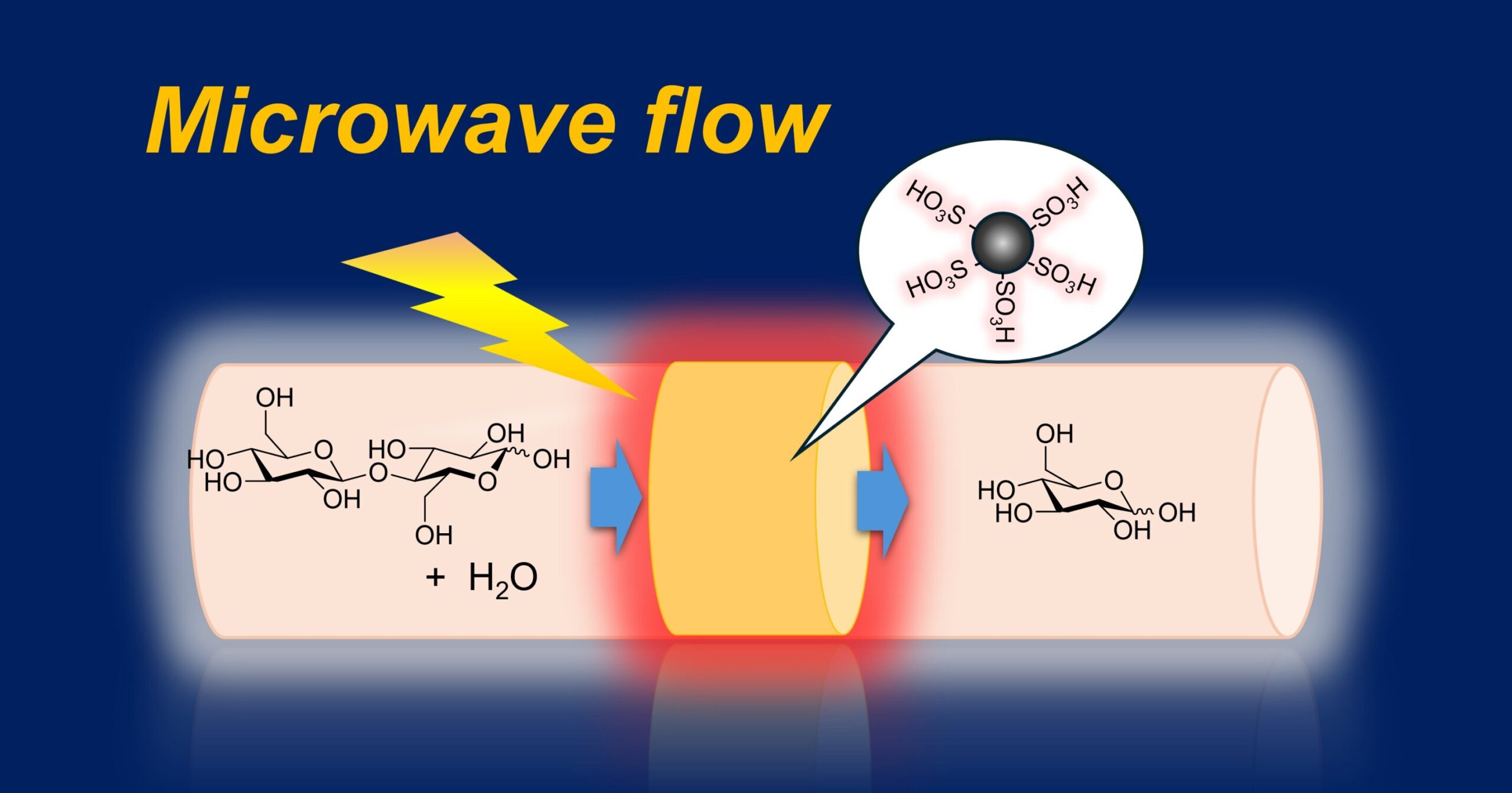
Researchers at Kyushu University have developed an innovative device that can efficiently convert complex polysaccharides into simple monosaccharides through the use of a catalyst and microwave-assisted flow reactions. This breakthrough technique combines cutting-edge science and engineering to address one of the significant challenges in the field of biomass conversion. The device employs a continuous-flow hydrolysis process in which cellobiose—a disaccharide composed of two glucose molecules—is passed through a sulfonated carbon catalyst heated using microwave energy. As a result, the cellobiose is broken down into glucose, an important monosaccharide that has various applications in industries such as food production, pharmaceuticals, and chemical synthesis.
Published in ACS Sustainable Chemistry & Engineering, the researchers’ findings promise to revolutionize how biomass polysaccharides, such as those found in wood, plants, and agricultural residues, are processed. By converting long-chain complex sugars into simpler, usable sugars, the device enhances the efficiency of a process that has been studied for decades. The demand for renewable, sustainable, and eco-friendly methods of biomass conversion is high, particularly as industries look for alternatives to petroleum-based products.
Biomass polysaccharides are attractive candidates for conversion because they are abundant, renewable, and can be utilized to produce a wide variety of valuable products. These sugars can be transformed into glucose or other simple sugars, which serve as building blocks in food, pharmaceuticals, and even industrial chemicals. One of the most efficient chemical processes to achieve this conversion is hydrolysis, where polysaccharides are broken down into smaller sugars by applying acid catalysts.
Traditionally, acid catalysts—whether in solid, liquid, or gas form—have been used to drive the hydrolysis reaction. While liquid or gas acid catalysts are widely used in industry, they have drawbacks. These catalysts tend to be challenging to recycle, limiting their sustainability. This has made solid acid catalysts, which can be more easily recycled, an area of interest for researchers seeking a more efficient and sustainable approach to biomass conversion. However, solid acid catalysts require high temperatures to operate efficiently, presenting an obstacle in optimizing the process for large-scale applications.
To tackle this challenge, Associate Professor Shuntaro Tsubaki, along with his team from Kyushu University’s Faculty of Agriculture, investigated the potential of combining solid acid catalysts with microwave heating to improve the reaction efficiency. Microwave-assisted reactions offer a unique advantage, as microwaves can provide rapid, targeted heating that enhances the catalytic activity of solid catalysts without needing high temperatures. This combination of technologies allows for a continuous flow system that ensures higher yields of the desired products.
Professor Tsubaki explains that microwaves create a localized high-temperature reaction field on the surface of the solid catalyst. This focused heat increases the catalytic activity while keeping the temperature of the overall system lower compared to conventional methods. The result is a more efficient reaction with improved yields. The key advantage lies in the ability to pass the substrate—a solution of cellobiose—through the heated solid catalyst, where the hydrolysis process takes place. This continuous flow allows the reaction to proceed more quickly and efficiently than static systems.
The microwaves in the system serve a dual purpose. The electric field in microwaves heats materials with dipolar characteristics, such as water molecules, which are essential in the dissolution of polysaccharides. At the same time, the magnetic field within the microwaves selectively heats conductive materials, including metals and carbon-based catalysts. By carefully separating these two fields, the researchers were able to fine-tune the heating process, optimizing the temperature and conditions for both the catalyst and the reactant solution. This dual-field approach increases the activity of the catalyst while also promoting the breakdown of the disaccharide into glucose.
The particular device developed by Tsubaki’s team utilizes a sulfonated carbon-based solid acid catalyst. In their experimental setup, cellobiose—a disaccharide—is passed through the sulfonated carbon catalyst, which has been preheated to temperatures between 100 and 140°C using microwaves. The sulfur groups on the carbon catalyst facilitate the hydrolysis process, splitting the cellobiose into two molecules of glucose. This efficient conversion process highlights the capability of the device to turn complex polysaccharides into high-value monosaccharides at high yield rates.
One of the unique aspects of the device is its ability to separately manage the interaction between the electric and magnetic fields produced by the microwaves. This separation enhances the reaction’s overall efficiency by focusing on specific heating targets—microwave heating for the substrate and magnetic field-induced heating for the catalyst. This innovative approach to combining microwave energy with solid acid catalysis opens new doors for developing efficient, renewable methods for biomass conversion.
Microwave-assisted catalytic reactions have been utilized for a variety of chemical reactions in organic synthesis, recycling processes, and now, biomass conversion. As the world shifts towards renewable energy sources, this type of electricity-driven chemical production could play a pivotal role in driving sustainable industrial practices and reducing reliance on fossil fuels.
In addition to glucose production, which is a key product for various industries, the researchers are exploring the broader potential applications of their methodology. Professor Tsubaki envisions their system contributing to more sustainable chemical processes, beyond just sugar conversion. For instance, the same principles could be applied to the hydrolysis of other polysaccharides or even proteins. The team believes their technology could be used to produce amino acids and peptides—compounds that have broad applications in the pharmaceutical, agricultural, and biotechnological industries.
Reference: Shuntaro Tsubaki et al, Efficient Cellobiose Hydrolysis over a Sulfonated Carbon Catalyst in a Spatially Separated Microwave Electric- and Magnetic-Field Flow Reactor, ACS Sustainable Chemistry & Engineering (2024). DOI: 10.1021/acssuschemeng.4c07690
Think this is important? Spread the knowledge! Share now.