Osteoarthritis (OA) is a degenerative joint disease that affects millions of people worldwide, particularly as they age. In OA, the cartilage that cushions the joints begins to deteriorate, leading to bone-on-bone friction, stiffness, pain, and in severe cases, disability. With more than 32 million people in the United States affected by this condition, osteoarthritis remains a major health concern.
Despite its prevalence, the progression of OA has been difficult to halt, with current treatments only addressing the symptoms rather than preventing the disease’s worsening. Traditional approaches such as nonsteroidal anti-inflammatory drugs (NSAIDs), physical exercise, weight loss, and lifestyle management remain pivotal in reducing pain and improving mobility for patients. However, none of these strategies can completely stop or reverse the progression of the disease.
The factors that contribute to the onset and advancement of osteoarthritis are varied. While wear-and-tear from aging is a well-known factor, other significant contributors include genetics, obesity, joint injuries, and even gender (with women being at a higher risk post-menopause). In fact, recent research suggests that genetics may account for nearly half the risk of developing OA, which has led to a heightened interest in unraveling the genetic underpinnings of the disease.
Genetic research into osteoarthritis offers the potential to develop more targeted, effective therapies. By identifying “causal” genes — those directly linked to the development and progression of the disease — scientists hope to uncover strategies to halt or even reverse cartilage degradation. One such effort is a groundbreaking study conducted by a multidisciplinary team of researchers at the UNC Thurston Arthritis Research Center (TARC). Led by Doug Phanstiel, Ph.D., Richard F. Loeser, M.D., and Brian Diekman, Ph.D., at the UNC School of Medicine, this team has made an important discovery that may pave the way for future treatments.
In a study published in Cell Genomics, the researchers identified 13 genes, termed “high probability risk genes,” that play a direct role in cartilage loss and significantly increase the chances of developing osteoarthritis. This marks a major advancement in our understanding of the genetic basis of OA, providing insights into potential targets for future drug therapies.
Before this study, hundreds of genes and genetic “risk regions” had been identified that appear to correlate with osteoarthritis, but these were largely preliminary findings that did not point directly to causation. In contrast, the UNC team’s findings offer a clearer understanding of how specific genes can contribute to the destruction of joint tissue. Loeser, who is also a professor of medicine at UNC, highlighted that while many genetic regions have been linked to osteoarthritis risk, only a handful of them were understood to directly influence the disease’s development.
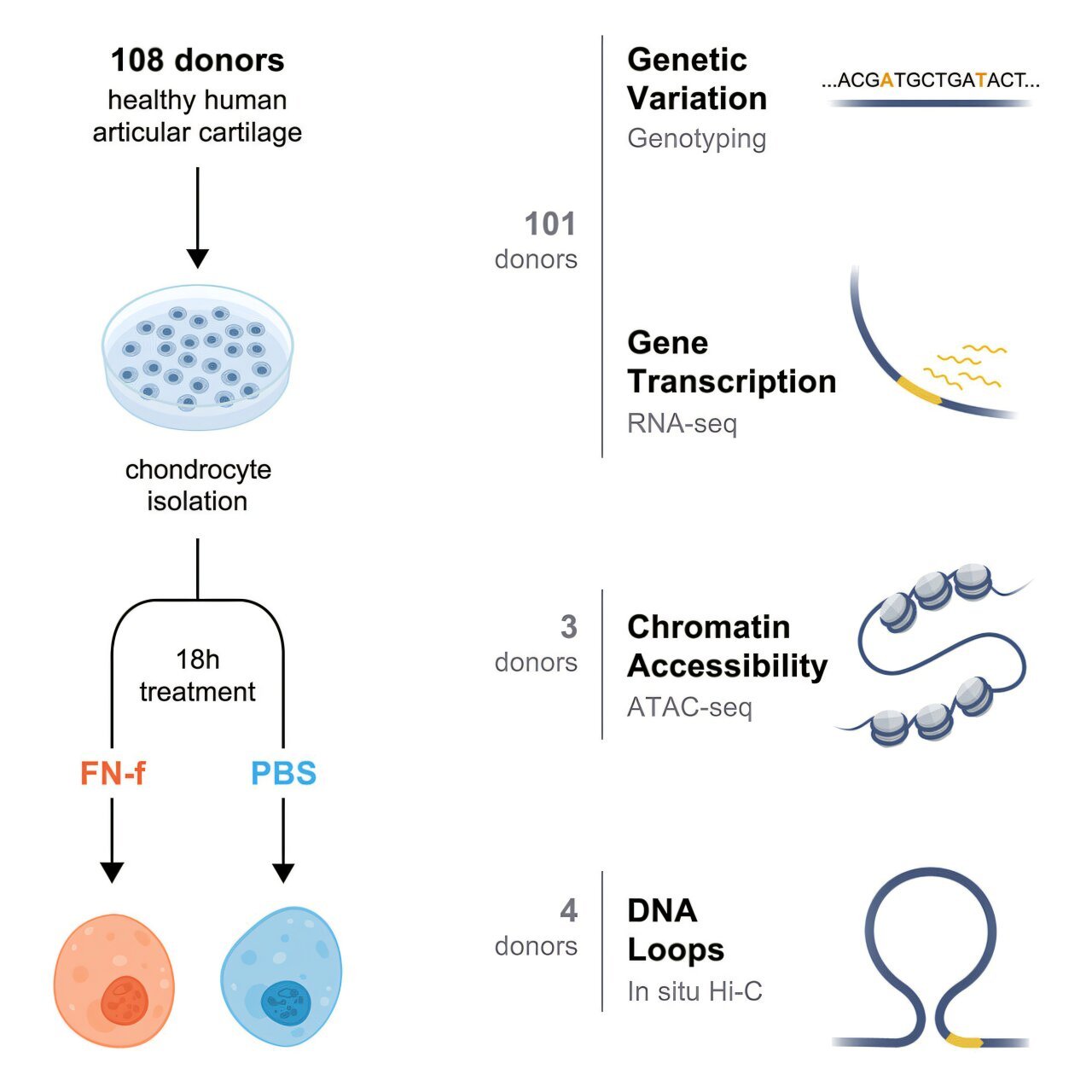
The team’s journey to uncovering these causal genes began back in 2022, when they set out to identify new risk factors that could be useful for therapy development. Loeser’s lab collected joint tissue samples from over 100 human donors, forming a cellular model of osteoarthritis in the lab. By mimicking osteoarthritis in these cells, they could study how the disease progresses in a controlled environment.
At this stage, Phanstiel brought his expertise in genomics to the project. As an associate professor of cell biology and physiology at UNC, Phanstiel’s lab focused on analyzing the gene expression profiles of the joint tissue samples. Using bioinformatics and computational tools, the team generated an extensive dataset that allowed them to pinpoint genes associated with osteoarthritis risk.
Next, Diekman, an expert in gene editing, joined forces to explore what role these genes played in disease progression. Through advanced gene-editing techniques, the team could modify and study the effects of the identified genes, further confirming their involvement in the disease process.
The final analysis revealed a set of 13 genes that play a crucial role in osteoarthritis risk. Six of these genes were completely novel, meaning that they had not been linked to osteoarthritis prior to this discovery. The remaining genes had known connections to the disease or were implicated in biological processes such as inflammation, cartilage degradation, and bone remodeling. In particular, some of the newly identified genes are involved in molecular pathways that govern the repair or breakdown of joint tissues, while others have roles in developmental biology or cellular signaling, which may explain their impact on OA. These findings offer hope that therapies targeting these specific pathways may prevent or slow down cartilage breakdown before it leads to debilitating joint damage.
Phanstiel emphasized that these newly identified genes help broaden our understanding of the biological processes at play in osteoarthritis. However, not all of the genes’ functions are completely understood, and their role in the disease is likely to vary across different individuals and disease stages. There’s also the possibility that osteoarthritis risk might not solely be driven by a single gene or pathway. Instead, it could result from the complex interaction of genetic influences acting across various stages of joint development and different biological conditions.
With this list of 13 high probability risk genes in hand, the researchers’ next steps are clear. They plan to expand their study by including a broader cohort of human donors to confirm and refine their findings further. Additionally, the team is working to explore the biological processes triggered by these newly discovered causal genes. To this end, researchers are examining how these genes affect cellular mechanisms such as inflammation, cartilage maintenance, and collagen production—key factors in the development of osteoarthritis.
Another exciting aspect of the study is the ongoing drug screening process. The researchers are testing various pharmaceutical compounds to see whether any can effectively target the pathways controlled by these risk genes. By identifying drugs that can modify the activity of these genes, the researchers hope to develop treatments that can prevent or delay the onset of osteoarthritis or even stop the disease from progressing at its earliest stages.
“The ultimate goal is to identify new therapeutics that will stop the damage to the joint before it causes pain or disability,” said Loeser. “By preventing the loss of cartilage early on, we could greatly improve the quality of life for individuals affected by osteoarthritis, helping them avoid the long-term consequences of the disease.”
If successful, this work could transform the future of osteoarthritis treatment. At present, no therapies are capable of reversing joint damage or stopping the disease from progressing in its later stages. By uncovering the genetic causes of osteoarthritis and identifying ways to modulate these genes, researchers could offer people living with the condition hope for treatments that not only manage symptoms but also address the root cause of the disease.
Reference: Nicole E. Kramer et al, Response eQTLs, chromatin accessibility, and 3D chromatin structure in chondrocytes provide mechanistic insight into osteoarthritis risk, Cell Genomics (2025). DOI: 10.1016/j.xgen.2024.100738