A recent study co-led by researchers from UCLA has unveiled a promising new avenue for combating glioblastoma, a highly aggressive and often fatal form of brain cancer. This discovery centers around a protein known as endocan and its related signaling pathway, offering a novel therapeutic approach that could not only hinder tumor growth but also make glioblastoma more susceptible to existing treatments like radiation therapy.
Glioblastoma is notorious for its complexity and resilience. It remains one of the most challenging types of cancer to treat, with a bleak prognosis. The average life expectancy following a glioblastoma diagnosis is a mere 12 to 15 months, and only about 5% of patients survive five years or more. A major contributing factor to this grim outlook is the tumor’s ability to evade standard therapies. One such factor is the way glioblastoma interacts with the blood vessel cells, also known as vascular endothelial cells, that supply the tumor with oxygen and nutrients. These tumor-associated blood vessels also secrete molecules that promote the survival of the cancer cells. A deeper understanding of this interaction has therefore become crucial in the search for better therapeutic options.
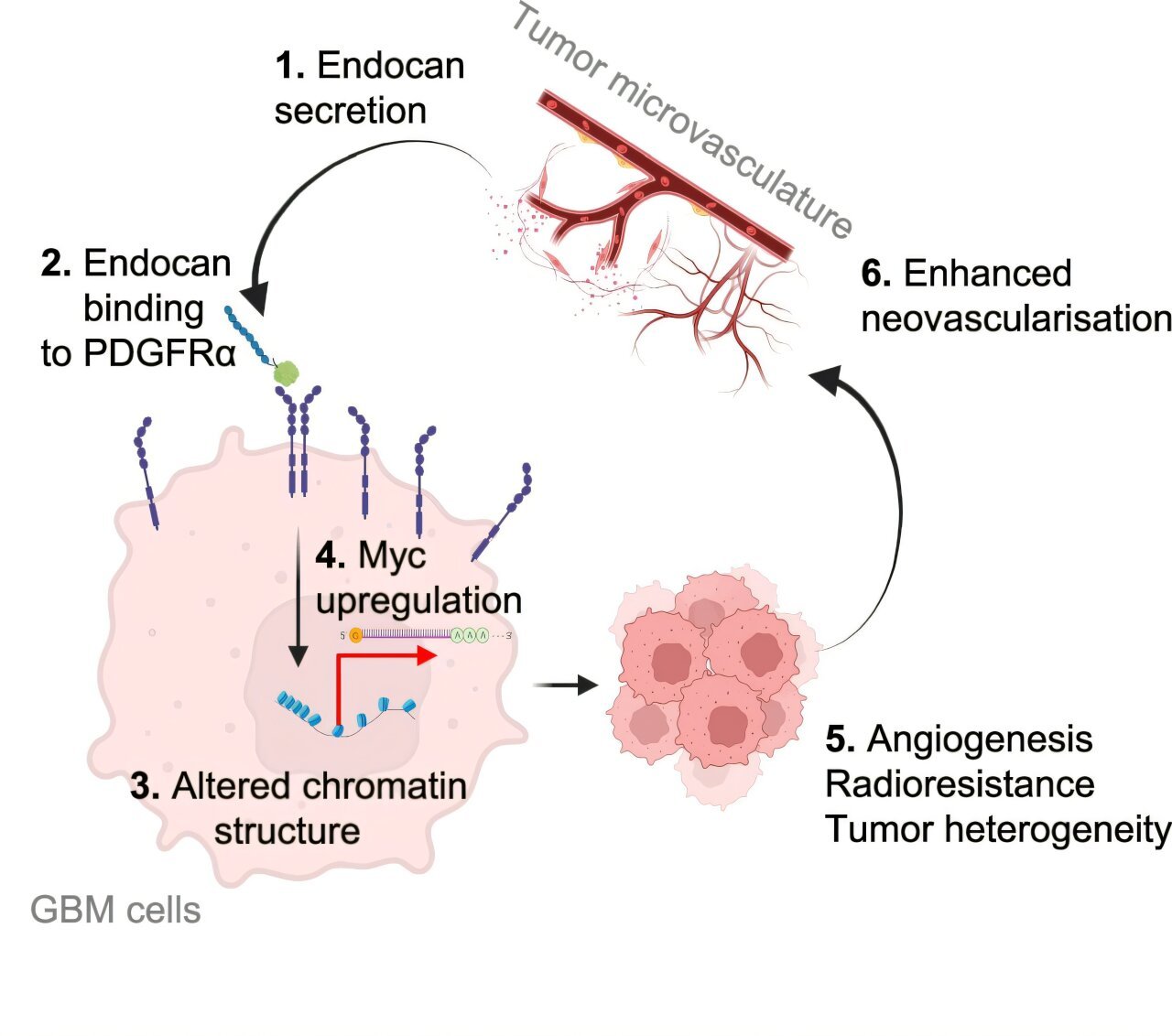
The study, published in Nature Communications, reveals that endocan, a protein produced by the endothelial cells lining the blood vessels in tumors, plays a pivotal role in driving the progression of glioblastoma. Researchers discovered that endocan activates a receptor called PDGFRA on glioblastoma cells, which in turn promotes tumor growth and confers resistance to traditional treatments such as radiation.
According to Dr. Harley Kornblum, the study’s co-senior author and the director of the UCLA Intellectual and Developmental Research Center, this discovery represents a major breakthrough in our understanding of glioblastoma. “By targeting the crosstalk between glioblastoma and vascular endothelial cells, we can develop treatments that prevent the tumor from adapting and surviving. This could also improve the effectiveness of treatments, especially radiation, making them more successful in tackling this aggressive cancer,” Kornblum emphasized.
The discovery that endocan activates PDGFRA opens the door to innovative therapeutic strategies. The traditional challenges faced when treating glioblastoma often arise from the tumor’s ability to adapt to environmental changes, which allows it to survive and evade targeted therapies. By targeting the interaction between endocan and PDGFRA, the researchers hope to disrupt the cancer’s ability to develop resistance to treatments, thereby improving the overall effectiveness of therapies like radiation.
To fully grasp how glioblastoma interacts with surrounding blood vessel cells, the research team relied on a database they developed in previous studies, which enabled them to identify the molecules present in tumor blood vessels and understand how they contribute to tumor progression. Endocan stood out as a key candidate molecule involved in this process, prompting further exploration into its specific role.
The research team employed a combination of experimental models, including glioblastoma cells, blood vessel cells derived from patients, genetically modified mice lacking endocan, and in vitro models, to investigate the functional implications of endocan. Their findings were striking. The team found that different regions of the tumor played distinct functional roles in tumor growth and behavior. More specifically, endocan was found to play a significant role in defining the geography of the tumor, particularly in the aggressive edge regions that often remain after surgical intervention. These edge regions are where glioblastoma cells frequently infiltrate healthy brain tissue, leading to tumor recurrence after surgery.
“Solving how tumors organize themselves is an important challenge,” said Kornblum, who is also a professor of psychiatry, pediatrics, and molecular and medical pharmacology at the David Geffen School of Medicine at UCLA. “While surgery can remove much of the tumor core, the infiltrative edge often remains following removal, leading to recurrence. Our research suggests endocan is a key player in this process, orchestrating both tumor cell behavior and the development of blood vessels that sustain tumor growth.”
Endocan’s role in glioblastoma does not end with tumor growth alone. The team also found that endocan interacts with PDGFRA, a receptor that plays a critical role in many cancers. By activating this receptor, endocan promotes tumor growth and contributes to the tumor’s resistance to radiation therapy, one of the standard treatment options for glioblastoma patients. In preclinical models, the researchers demonstrated that blocking the interaction between endocan and PDGFRA using a targeted therapy drug, ponatinib, significantly extended survival and enhanced the response to radiation therapy.
These preclinical results highlight the potential for using endocan as a therapeutic target in glioblastoma. By disrupting the signaling pathway between endocan and PDGFRA, scientists could develop new treatments that not only curb tumor growth but also increase the efficacy of existing therapies.
The study also identified an important connection between endocan’s effects and a protein called cMyc, which is critical in the development and progression of many cancers, including glioblastoma. However, cMyc is notoriously difficult to target directly with therapeutic drugs. According to Kornblum, inhibiting the endocan-PDGFRA axis may provide an indirect but effective means of disrupting cMyc’s role in glioblastoma. This insight offers hope for overcoming one of the major hurdles in targeting this protein for therapeutic intervention.
As the study moves forward, researchers plan to validate their findings in human glioblastoma tumors, particularly in the cells located at the infiltrative edge where the tumor tends to resist treatment and regrow. Additionally, the team will focus on exploring whether targeting endocan can enhance responses to radiation therapy in clinical settings, making this approach a potentially transformative treatment for glioblastoma patients.
The study was co-authored by Dr. Ichiro Nakano from Harada Hospital in Japan, and the primary authors were Soniya Bastola and Marat Pavlyukov from UCLA. Together, this collaborative team’s work offers significant hope for a novel approach to treating glioblastoma, a cancer that has long been one of the most difficult to treat.
Glioblastoma presents one of the greatest challenges in oncology, and this research opens up promising avenues for targeted therapies that could improve survival rates and enhance the effectiveness of existing treatments like radiation. If these findings are validated in clinical settings, we could see the development of therapies that not only halt tumor growth but also transform the prognosis for those diagnosed with this aggressive form of brain cancer. The implications of this study could lead to breakthrough treatments that dramatically improve the outcomes for glioblastoma patients worldwide, signaling a potential turning point in the fight against one of the deadliest cancers.
Reference: Soniya Bastola et al, Endothelial-secreted Endocan activates PDGFRA and regulates vascularity and spatial phenotype in glioblastoma, Nature Communications (2025). DOI: 10.1038/s41467-024-55487-1
Think this is important? Spread the knowledge! Share now.