Microorganisms have been using hydrogen as a source of energy for billions of years, utilizing specialized enzymes known as hydrogenases. These enzymes play a crucial role in hydrogen metabolism by catalyzing the conversion of hydrogen molecules into protons and electrons. Hydrogenases are made up of metal-containing catalytic centers, and understanding the detailed mechanism by which these enzymes catalyze hydrogen conversion is a key area of research. This knowledge could pave the way for the development of more efficient biocatalysts for hydrogen production and conversion, contributing to sustainable energy solutions.
In a significant breakthrough, a collaborative team from multiple prestigious institutions, including the Max Planck Institutes (MPI), the Center for Biostructural Imaging of Neurodegeneration (BIN) at the University Medical Center Göttingen (UMG), the University of Kiel, and FACCTs GmbH, has made new strides in the understanding of hydrogenases. This international team used an innovative approach to amplify signals in magnetic resonance spectroscopy, allowing them to observe previously unseen intermediate steps in the hydrogen conversion process. The study, published in Nature Catalysis, offers new insights into the catalytic cycles of [Fe] hydrogenases, a class of hydrogenases that had not been fully understood until now.
Hydrogen is increasingly being viewed as a key player in the transition to a sustainable energy future. As an energy source, hydrogen is seen as a potential replacement for fossil fuels, as it produces no carbon dioxide (CO₂) when burned or used in fuel cells. Hydrogen can also act as a catalyst in chemical processes, further adding to its appeal as a green alternative. However, hydrogen does not naturally exist in its pure molecular form on Earth. Instead, it is primarily found as part of other compounds, such as water (H₂O), fossil fuels like natural gas and oil, and organic materials. To harness pure hydrogen, energy is required to break it free from these compounds through a process known as hydrogen production.
Currently, the most common method of producing hydrogen is steam methane reforming (SMR), which involves extracting hydrogen from natural gas. However, this process also produces carbon dioxide (CO₂), a potent greenhouse gas, making it less environmentally friendly. Alternatively, electrolysis is used to produce hydrogen by splitting water molecules into hydrogen and oxygen, often using platinum-based electrodes. However, this method is still relatively expensive due to the high cost of platinum, which is a precious metal.
In contrast, many microorganisms have evolved more efficient and sustainable ways of generating hydrogen. These organisms use hydrogenases to catalyze the production of hydrogen without the need for precious metals or the release of CO₂. There are three main types of hydrogenases found in nature: [NiFe] hydrogenases, [FeFe] hydrogenases, and [Fe] hydrogenases. The [NiFe] and [FeFe] hydrogenases are found in a variety of bacteria and archaea, while [Fe] hydrogenases are mostly found in archaea and play a key role in methanogenesis, the process by which carbon dioxide is reduced to methane.
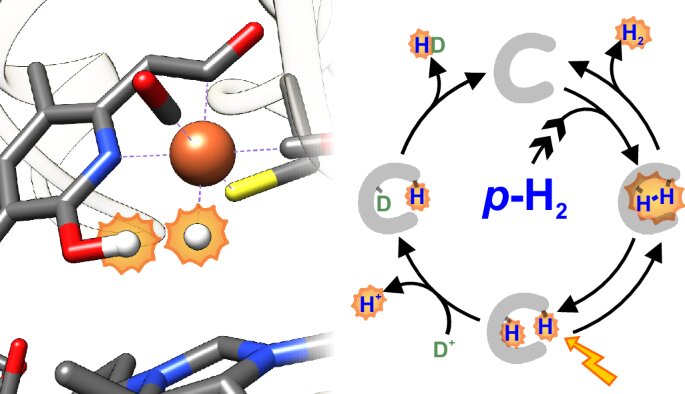
While much is known about the catalytic cycles of [NiFe] and [FeFe] hydrogenases, the intermediates involved in the catalytic cycle of [Fe] hydrogenases have remained elusive. The [Fe] hydrogenases, unlike their counterparts, contain a homodimeric structure with an iron center in each subunit. This iron center is coordinated by a unique cofactor called guanylylpyridinol, which plays an essential role in hydrogen binding and conversion. Until recently, scientists had been unable to directly observe the intermediates involved in the catalysis of these enzymes.
The breakthrough by the research team from the Max Planck Institutes and their collaborators addresses this gap in knowledge. By using a novel technique that exploits the chemical properties of hydrogen, the team was able to visualize the reaction intermediates of the [Fe] hydrogenase catalytic cycle for the first time. The key to this discovery lay in the different forms that hydrogen can take, specifically in terms of its nuclear spin. Hydrogen exists in two distinct forms: parahydrogen and orthohydrogen. These two forms differ in the alignment of the spins of their nuclei, and this difference can be exploited to enhance the signals obtained from magnetic resonance spectroscopy.
The researchers demonstrated that when [Fe] hydrogenase reacts with parahydrogen, the resulting nuclear magnetic resonance (NMR) signals are amplified due to a phenomenon known as parahydrogen-induced polarization (PHIP). This amplification made it possible to observe the previously undetectable intermediates involved in the hydrogen conversion process. The team was able to identify key steps in the reaction mechanism, including the formation of a hydride at the iron center of the enzyme, which is crucial for the enzyme’s ability to bind and transfer hydrogen.
One of the key advantages of the PHIP technique is its sensitivity, which allows for the detection of reaction intermediates in extremely small quantities. This makes it an ideal tool for studying biological reactions in living cells, as it enables researchers to investigate hydrogen metabolism in vivo. The ability to visualize hydrogen conversion at this level of detail could lead to new approaches for optimizing the catalytic efficiency of hydrogenases, making them more effective for industrial applications in hydrogen production and energy storage.
In addition to providing valuable insights into the mechanism of [Fe] hydrogenases, this study has broader implications for the development of sustainable hydrogen production technologies. By gaining a deeper understanding of how microorganisms catalyze hydrogen conversion, researchers can apply this knowledge to the design of bioinspired catalysts that are more efficient and cost-effective than current methods. The ultimate goal is to create systems that can produce hydrogen in a way that is both environmentally friendly and economically viable, which is crucial for the future of clean energy.
This study also opens up new possibilities for investigating hydrogen metabolism in living organisms. Hydrogen is an important energy carrier in many microbial communities, particularly in anaerobic environments where other sources of energy may be scarce. By studying how hydrogenases function in these environments, scientists can gain insights into the role of hydrogen in microbial ecology and its potential applications in bioremediation, bioenergy production, and even synthetic biology.
The discovery of new intermediates in the catalytic cycle of [Fe] hydrogenases is just one example of how cutting-edge techniques in spectroscopy and structural biology are transforming our understanding of biological catalysis. As researchers continue to explore the complexities of hydrogenases and other biocatalysts, we can expect to see further advances in the field of hydrogen production, offering new solutions to some of the most pressing challenges of our time, including the need for clean, renewable energy sources and the reduction of greenhouse gas emissions.
In conclusion, the work done by the team from the Max Planck Institutes and their collaborators represents a significant advancement in our understanding of hydrogenases and the catalytic processes that underlie hydrogen conversion. By utilizing parahydrogen-induced polarization to visualize reaction intermediates, the researchers have opened up new avenues for the development of bioinspired catalysts that could revolutionize hydrogen production. The results of this study are an important step forward in the quest for sustainable energy solutions and have the potential to accelerate the transition to a cleaner, more sustainable energy economy.
Reference: Lukas Kaltschnee et al, Parahydrogen-enhanced magnetic resonance identification of intermediates in the active [Fe]-hydrogenase catalysis, Nature Catalysis (2024). DOI: 10.1038/s41929-024-01262-w. www.nature.com/articles/s41929-024-01262-w
Think this is important? Spread the knowledge! Share now.