Scientists at the Children’s Medical Research Institute (CMRI) have made a groundbreaking discovery in the field of cancer research that promises to reshape cancer treatment and enhance therapeutic outcomes. This breakthrough, which addresses a critical mystery in how cells die following radiation therapy, provides fresh insights that could pave the way for more effective cancer treatments and improved survival rates for patients undergoing radiotherapy.
For decades, scientists have struggled to understand why cells within the same tumor respond differently to radiation therapy. This phenomenon is particularly significant in cancer treatment because the way a cell dies after radiotherapy directly impacts the patient’s immune response. Some types of cell death are invisible to the immune system, whereas others can trigger an immune response that aids in killing additional cancer cells. By harnessing the power of the immune system to target and destroy tumors, researchers hope to greatly improve treatment efficacy and cure rates.
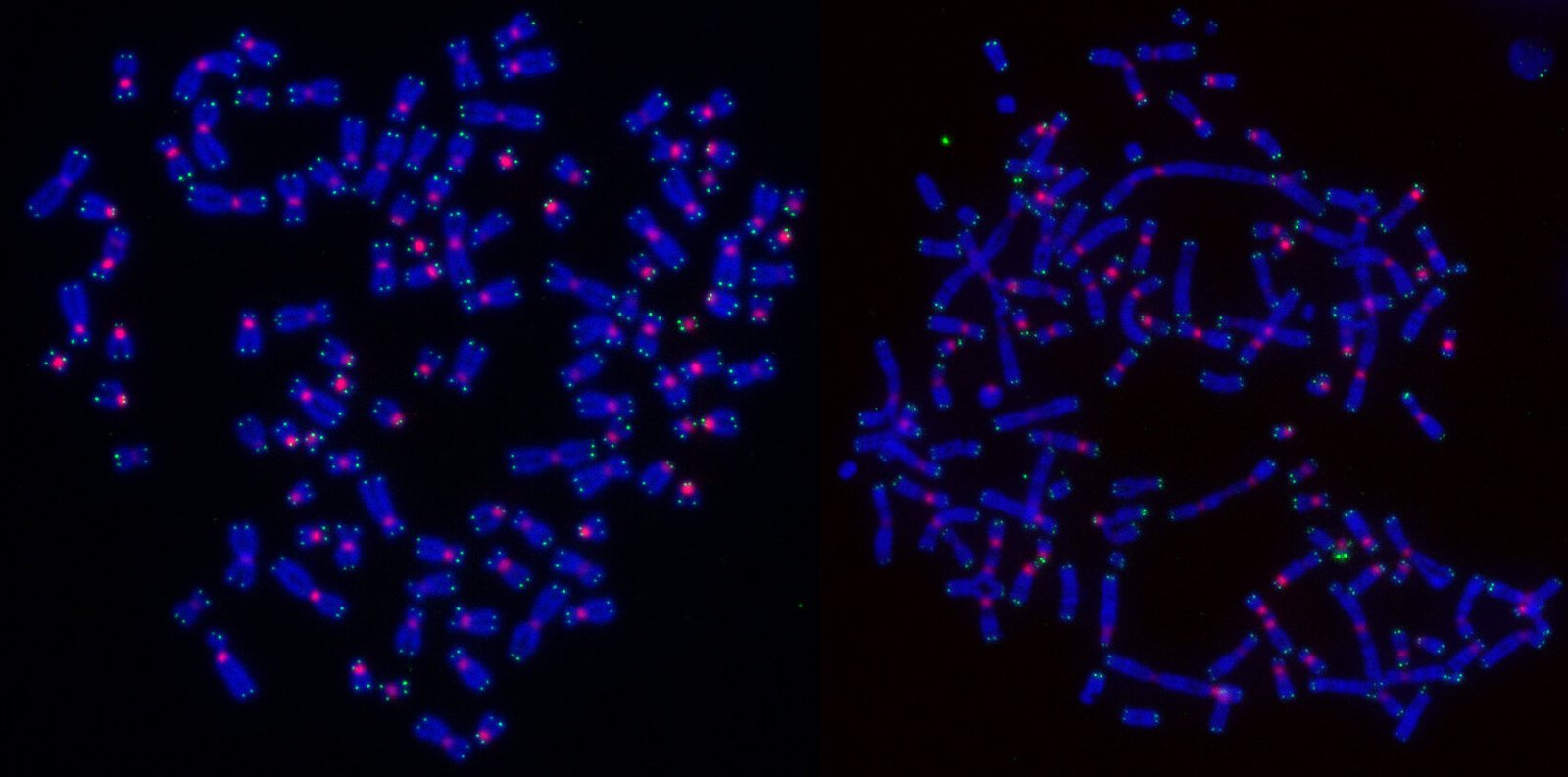
The recent findings from CMRI, published in Nature Cell Biology, bring scientists one step closer to this goal. Led by Professor Tony Cesare and carried out by first author Dr. Radoslaw Szmyd at CMRI’s Genome Integrity Unit, the study explores the mechanisms behind how radiation therapy causes cancer cells to die and the surprising role that DNA repair plays in this process. Dr. Szmyd and his team uncovered a key piece of the puzzle: the DNA repair processes that typically protect healthy cells from damage may actually influence the way cancer cells die in response to radiotherapy.
Radiotherapy is a critical cancer treatment that uses ionizing radiation to target and destroy cancer cells. However, radiation can also damage healthy cells, and scientists have long been puzzled about how tumor cells, which are exposed to the same radiation, react so differently. Some cancer cells seem to be killed without any noticeable effect on the immune system, while others produce signals that alert the immune system to their presence, prompting a cascade of immune activity aimed at eradicating both the targeted cancer cells and nearby tumor cells.
According to Professor Cesare, this new discovery indicates that DNA repair processes, which are typically activated to safeguard the integrity of a cell’s DNA, play an essential role in determining how a cancer cell responds to radiotherapy. “DNA repair, which normally protects healthy cells, can, in the context of radiation therapy, actually instruct cancer cells on how to die,” explained Professor Cesare. This discovery has significant implications for the design of more targeted cancer treatments.
To understand why cancer cells die differently following radiation, the researchers focused on the process of DNA repair. DNA inside all living cells is constantly exposed to damage caused by factors such as UV light, chemicals, and, importantly, radiation from treatments like radiotherapy. To prevent this damage from causing long-term harm, cells have developed complex repair mechanisms. The most efficient of these mechanisms, called homologous recombination, is particularly vital when it comes to fixing DNA double-strand breaks caused by radiation.
However, the study revealed that when cancer cells repair radiation-damaged DNA via homologous recombination, they die in a way that evades detection by the immune system. This process leads to cell death during cell division, a stage known as mitosis, and because this form of cell death goes undetected, it does not trigger an immune response. This outcome is not desirable in cancer treatment because it deprives the immune system of the opportunity to attack cancer cells that survived the radiation treatment.
In contrast, cancer cells that repair radiation-induced DNA damage using alternative DNA repair pathways are not killed during cell division. Instead, these cells survive and release byproducts of the repair process into the cell’s interior. To the cell, these repair byproducts resemble a viral or bacterial infection, prompting the immune system to recognize the damaged cell and launch an immune response. This type of immune activation is highly beneficial, as it enables the body’s defense mechanisms to recognize and destroy the cancer cells, helping to clear the tumor more effectively.
Building on this key finding, the researchers were able to show that blocking homologous recombination in cancer cells shifted the manner of cell death, making it more likely to alert the immune system. By altering the way cells die, the treatment could stimulate the immune system to detect and attack remaining cancer cells. This opens up exciting new possibilities for improving the immune response to radiotherapy, potentially enhancing the effectiveness of radiation treatment and boosting survival rates.
The researchers also found that certain mutations in the BRCA2 gene, which is critical for the homologous recombination process, can alter the response to radiation. Mutations in BRCA2 are well-known to be associated with certain types of breast cancer, as well as other cancers, and are linked to defects in DNA repair. The study showed that cancer cells with a mutation in BRCA2 did not die during cell division following radiation treatment. Instead, they survived and could potentially spread in the absence of an immune response, highlighting the complexity of how different tumors react to radiotherapy based on their genetic makeup.
Dr. Szmyd, who worked on this research for six years, described the findings as a major breakthrough in the field. “This incredibly difficult question has been something we’ve been working on for years, and to see it pay off in a way that can directly impact patient care is very rewarding,” said Dr. Szmyd. His persistence, along with the collaboration of the research team, has opened new avenues for treating cancers, particularly in how radiotherapy can be combined with other therapies like immunotherapy for enhanced effect.
The research holds particular promise for developing combination therapies that target the DNA repair pathways alongside radiotherapy to improve cancer treatment. These findings could allow for the development of drugs that block homologous recombination, forcing cancer cells treated with radiotherapy to die in a way that triggers the immune system’s response, thereby enhancing the body’s natural ability to target and destroy cancer.
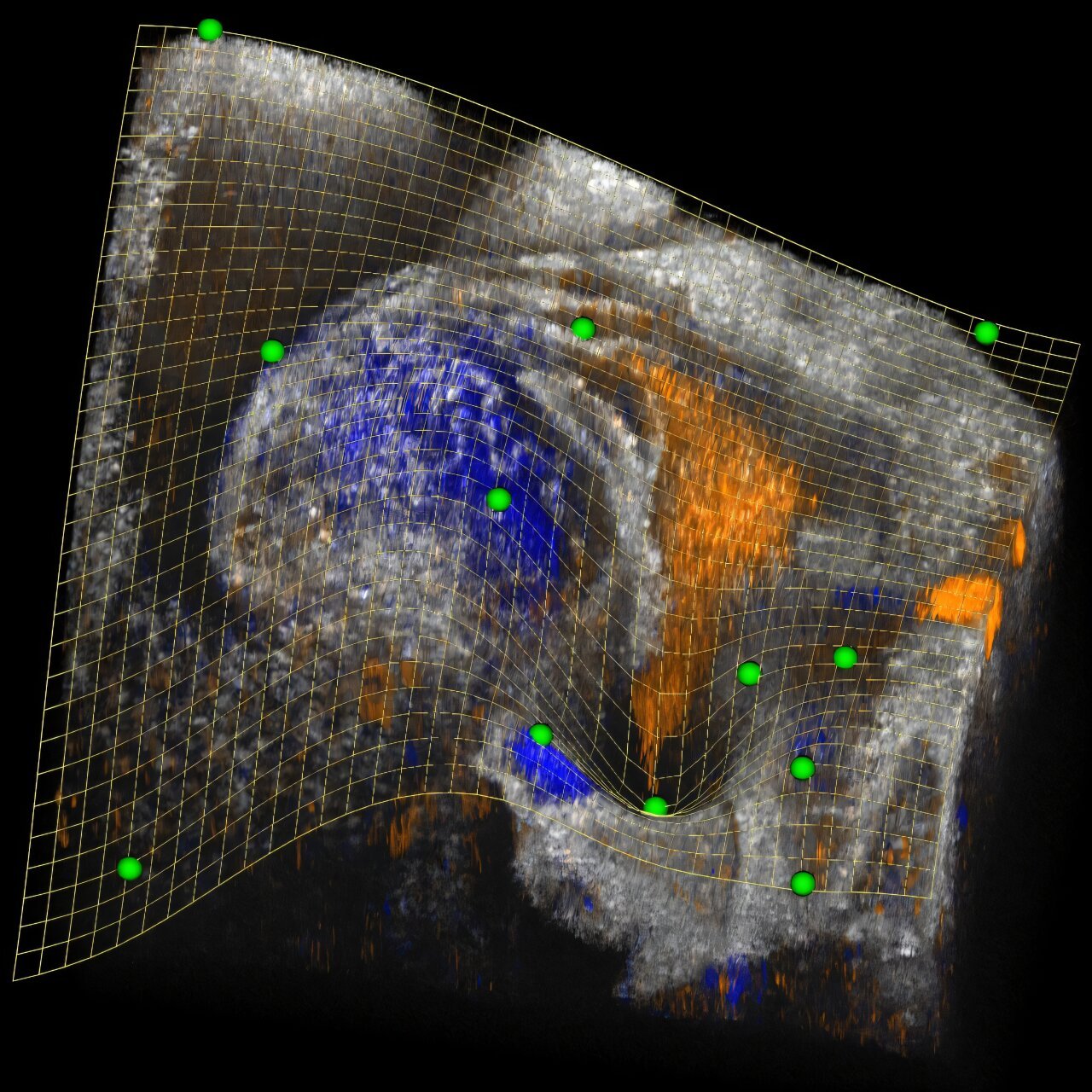
In addition, the team used advanced live cell microscopy technology to track the fate of irradiated cancer cells over the course of a week. This imaging technique provided an unprecedented view of the various ways in which radiation-induced damage to cancer cells can unfold. “Live imaging gave us a deeper understanding of the complex outcomes following radiation therapy and helped us pinpoint exactly how cancer cells respond,” said Prof. Cesare.
These findings are especially relevant for clinicians and oncologists. A/Prof. Harriet Gee, a radiation oncologist at the Western Sydney Local Health District Radiation Oncology Network and co-project lead, commented on how these insights can directly improve cancer treatment. “We’ve answered a question that has puzzled the field for 30 years—understanding how specific DNA repair pathways influence the way tumor cells die after high-dose, targeted radiation,” she said. “This discovery opens up new possibilities for enhancing radiation therapy through the addition of immunotherapy, which could lead to better outcomes and more successful cancer cures.”
Looking to the future, these insights may be the foundation for creating new treatment strategies that leverage both radiotherapy and immune-based therapies. By targeting specific DNA repair mechanisms, clinicians could potentially make cancers more responsive to radiation, sparking stronger immune reactions that clear tumors more effectively and reducing the risk of cancer recurrence.
This research marks a significant step forward in the ongoing fight against cancer, offering new hope for patients and revolutionizing the way doctors approach cancer therapy. It showcases how combining basic scientific discoveries with cutting-edge technology and clinical expertise can lead to innovative solutions in the field of cancer treatment, ultimately saving lives and improving health outcomes for millions of people worldwide.
The study involved contributions from CMRI researchers Sienna Casolin, Lucy French, Dr. Anna Gonzalez-Manjon, Dr. Melanie Walter, Lea Cavalli, Scott Page, Prof. Hilda Pickett, Dr. Christopher Nelson, and Dr. Andrew Dhawan, along with A/Prof. Eric Hau from the Westmead Clinical School at the University of Sydney. Through the efforts of these dedicated scientists, a critical piece of the puzzle regarding cancer cell death and immune responses has been uncovered, offering exciting new directions for cancer research and patient care.
Reference: Homologous recombination promotes non-immunogenic mitotic cell death upon DNA damage, Nature Cell Biology (2025). DOI: 10.1038/s41556-024-01557-x
Love this? Share it and help us spark curiosity about science!