An interdisciplinary team of researchers from POSTECH has developed a pioneering technology that significantly enhances clean hydrogen production using microwaves. Their recent findings, published on the inside front cover of the Journal of Materials Chemistry A, highlight a major breakthrough that could have a transformative impact on sustainable energy. This new approach addresses many of the limitations faced by existing hydrogen production methods and unlocks new avenues for energy-efficient hydrogen generation.
The Growing Need for Clean Hydrogen
As the world looks for ways to transition away from fossil fuels, clean hydrogen has emerged as one of the most promising candidates for a zero-carbon energy future. Hydrogen is widely considered the fuel of the future due to its ability to generate electricity with water as the only byproduct. However, despite its potential, the production of hydrogen at scale still faces a number of technical and economic hurdles.
Most conventional methods for hydrogen production involve thermochemical reactions, particularly the oxidation-reduction of metal oxides, which requires extremely high temperatures—sometimes reaching up to 1,500°C. These methods are not only energy-intensive but also economically impractical due to the immense cost of heating materials to such high temperatures. Moreover, achieving scalability in these processes is challenging, which makes their application in large-scale commercial production inefficient.
The Role of Microwaves in Hydrogen Production
In their quest to find a more efficient way of producing hydrogen, the POSTECH team turned their attention to an energy source that has long been utilized for other applications: microwaves. Known to most people as the energy source used in household microwave ovens, microwaves are typically associated with food heating. However, researchers have begun to realize their potential in driving chemical reactions efficiently and cost-effectively.
Microwaves can penetrate materials and interact with the molecules inside them, making them effective at providing localized heating. Unlike traditional heating methods that rely on external heat sources, microwaves work by directly exciting the molecules involved in the reaction. This leads to faster and more efficient reactions at much lower temperatures than conventional methods.
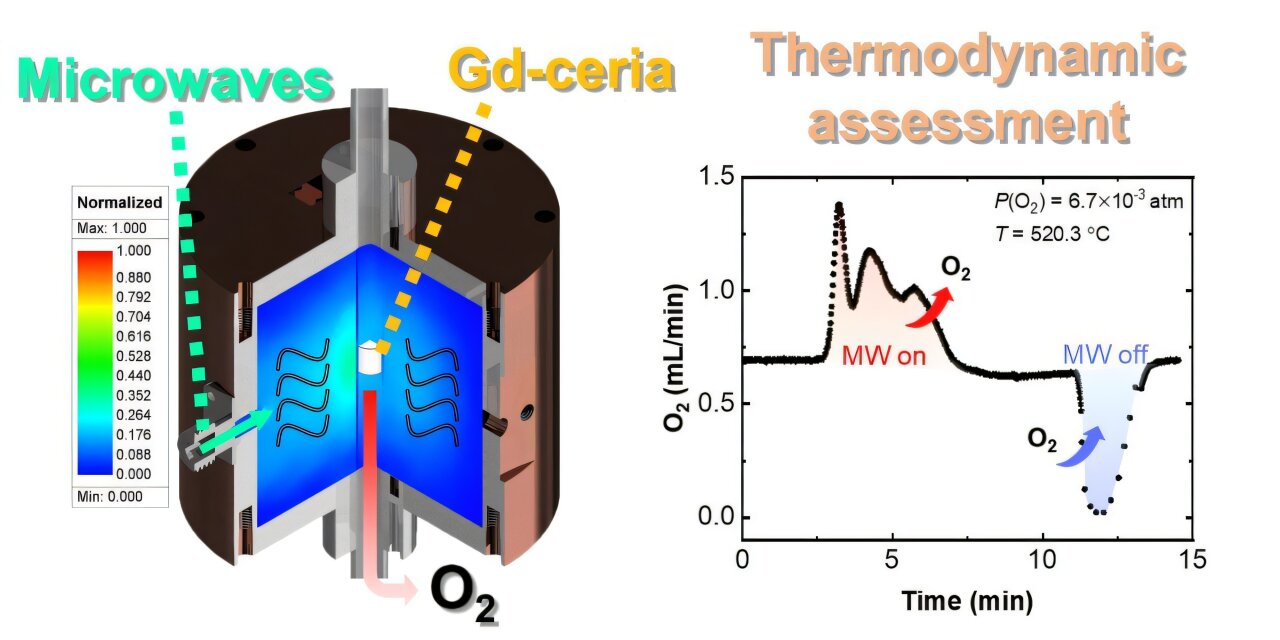
In this groundbreaking research, the POSTECH team applied microwave technology to enhance the production of hydrogen from Gd-doped ceria (CeO2), a commonly used material for thermochemical hydrogen production. Traditionally, high temperatures—often exceeding 1,500°C—are required to drive the reaction and produce hydrogen. The POSTECH team’s novel approach demonstrated that by using microwave energy, the reduction temperature of Gd-doped ceria could be reduced to below 600°C, marking an impressive 60% reduction in the temperature requirement.
Energy Efficiency Through Microwave Heating
One of the most significant achievements of this research was the discovery that microwave energy could replace up to 75% of the thermal energy traditionally needed in the reduction reaction. This drastic reduction in energy requirements could lead to both lower operational costs and less energy waste, making hydrogen production more economically viable.
By utilizing microwaves, the team was able to lower the activation energy of the reaction, enhancing both the efficiency and feasibility of hydrogen production. This is an exciting development as it not only lowers the environmental footprint of the process but also opens the door for more widespread adoption of hydrogen production methods that are less reliant on energy-intensive operations.
The Formation of Oxygen Vacancies
In addition to reducing the overall temperature requirement for the reduction process, the POSTECH team’s research also identified an innovative way of forming oxygen vacancies—defects within the structure of the ceria material—required for effective hydrogen production.
Under conventional methods, creating these oxygen vacancies is a slow process that takes several hours at extremely high temperatures. However, the team discovered that microwave energy could form oxygen vacancies in a matter of minutes, even at temperatures as low as 600°C. This breakthrough in speed and efficiency could be vital in improving the scalability of hydrogen production.
To validate these findings, the researchers developed a thermodynamic model that helped explain the mechanism behind the microwave-driven reaction. The model provided further insight into how the introduction of microwave energy can enhance the chemical process, effectively speeding up reactions and enabling higher yields of hydrogen in less time.
Revolutionizing Thermochemical Hydrogen Production
Professor Hyungyu Jin, one of the leading researchers in this project, expressed excitement about the implications of the research, stating, “This research has the potential to revolutionize the commercial viability of thermochemical hydrogen production technologies. By significantly reducing the energy required and improving efficiency, we can pave the way for large-scale hydrogen production systems that are more sustainable and cost-effective.”
The use of microwave technology is particularly significant in this context because it eliminates one of the primary obstacles in hydrogen production: the high energy costs associated with achieving the required temperatures. By significantly lowering the temperatures needed for the reaction, the POSTECH team’s microwave-driven method could unlock new efficiencies, making hydrogen a more attractive option in the transition to clean energy.
A Collaborative Breakthrough
The success of this innovative approach can also be attributed to the interdisciplinary collaboration among the team members. Professor Gunsu Yun emphasized the importance of the teamwork that made the breakthrough possible: “Introducing a new mechanism powered by microwaves and overcoming the limitations of existing processes are major achievements, made possible through the close interdisciplinary collaboration of our research team.”
This collaboration between researchers with expertise in materials science, physics, engineering, and chemistry exemplifies how interdisciplinary approaches can lead to breakthroughs that transcend traditional academic boundaries. By bringing together diverse perspectives and knowledge, the POSTECH team was able to develop a method that is not only more efficient but also potentially scalable for industrial applications.
Towards a Sustainable Energy Future
With the ability to efficiently produce hydrogen at lower temperatures and reduce energy consumption, POSTECH’s technology could significantly accelerate the development of clean hydrogen as a widely accessible energy source. This achievement represents a crucial step forward in addressing the global energy crisis and moving toward a more sustainable energy future.
The research published by the team sets the stage for the next generation of hydrogen production technologies that could power homes, industries, and transportation systems with zero-carbon emissions. As the world seeks to reduce its dependence on fossil fuels, advances like this one provide hope that sustainable energy solutions are not only feasible but within reach.
Conclusion
The POSTECH team’s groundbreaking use of microwave technology to enhance clean hydrogen production represents a major advancement in sustainable energy research. By reducing the temperature requirement for hydrogen production by over 60% and replacing up to 75% of the thermal energy traditionally needed, this innovative approach offers significant improvements in both energy efficiency and cost-effectiveness. The ability to create oxygen vacancies rapidly at lower temperatures further accelerates the process, making it more scalable and commercially viable. This research not only addresses key limitations of conventional hydrogen production methods but also opens the door for the development of new, microwave-optimized materials for a range of chemical processes. As the world transitions toward cleaner energy sources, these breakthroughs hold the potential to make hydrogen a cornerstone of future energy solutions, contributing to a more sustainable and economically feasible energy landscape. This work marks a pivotal step toward a greener, hydrogen-powered future.
Reference: Dongkyu Lee et al, Thermodynamic assessment of Gd-doped CeO2 for microwave-assisted thermochemical reduction, Journal of Materials Chemistry A (2024). DOI: 10.1039/D4TA05804F