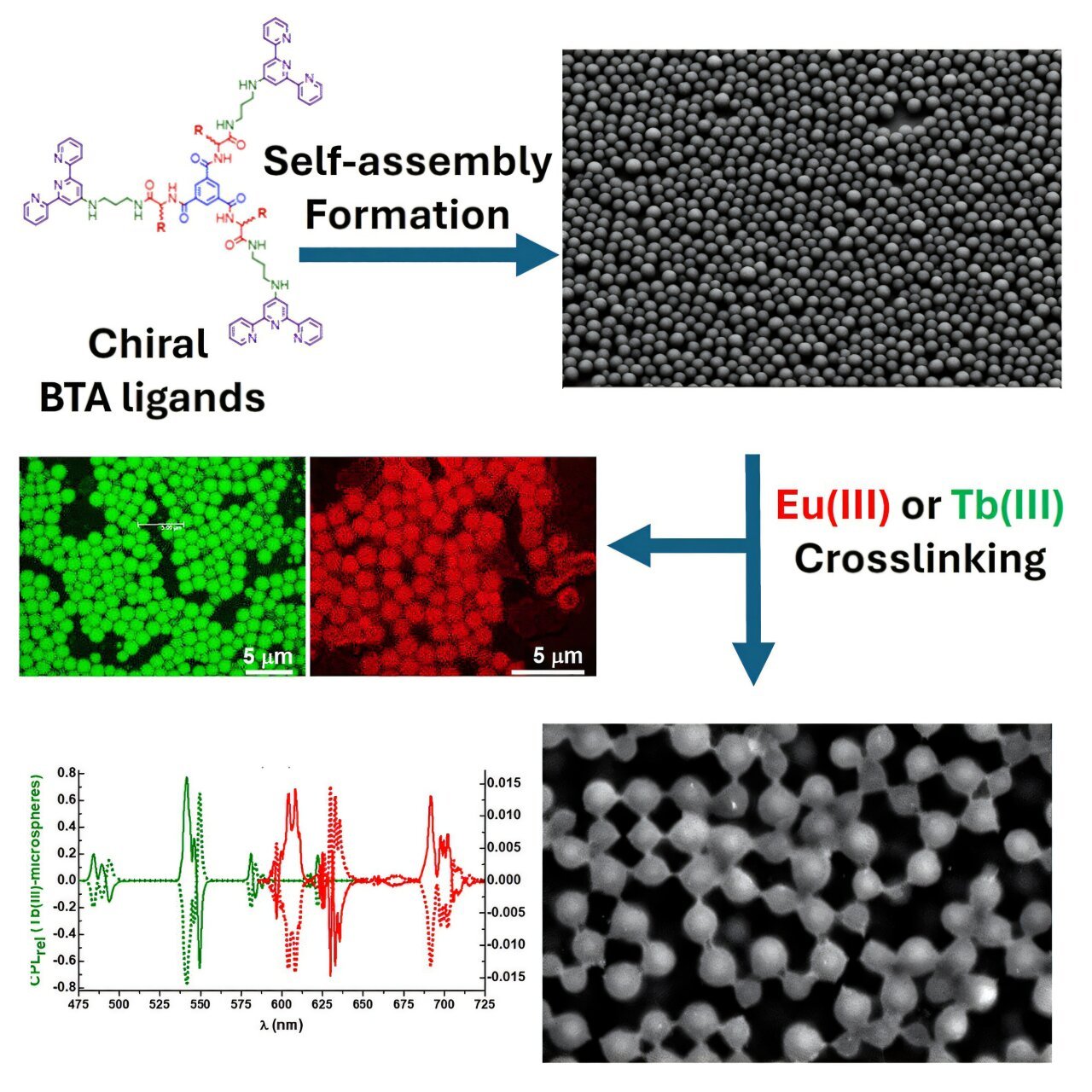
In a remarkable step forward in chemistry, scientists have made groundbreaking strides in programming molecules to self-assemble in specific, controlled, and predictable ways. The research could pave the way for future innovations in various fields, including highly sensitive sensors, drug delivery systems, and other high-tech applications that harness the power of molecular self-assembly.
The self-assembly of molecules—a fundamental process that occurs in nature—is at the heart of how biological systems function. Cells, enzymes, and various other biological components demonstrate extraordinary precision in the way they organize themselves to carry out essential tasks required for survival. Despite such successes in the natural world, the underlying mechanisms that govern self-assembly remain elusive to researchers. Scientists have long struggled to decipher these mechanisms and to replicate them in the laboratory to achieve consistent, reproducible, and desirable molecular outcomes.
This gap in understanding presents both a significant challenge and an incredible opportunity. If chemists can master the art of programming molecules to self-assemble in predictable ways, they could potentially design molecules that perform specific tasks with unparalleled precision. Recent advances in this area have taken us closer to realizing these ambitious goals, as revealed in a groundbreaking study published in the journal Chem.
The Chemistry Behind Malteser-like Molecules
The study was conducted by a multidisciplinary team of scientists from Trinity College Dublin, working in collaboration with researchers from Durham University. The research is led by Professor Thorfinnur Gunnlaugsson, a distinguished figure at the Trinity Biomedical Sciences Institute (TBSI), alongside Professor John Boland from CRANN. Their collaborative effort involved the contributions of several key scientists, including Aramballi Savyasachi, the study’s first author and a former Ph.D. student at Trinity.
The team developed a fascinating set of molecules with remarkable self-assembly characteristics, inspired by the shape of Maltesers, the British chocolate malted milk candy. These “Malteser-like” molecules exhibit a unique ability to form spherical structures, made possible by the specific amino acid sequences that serve as their building blocks.
Amino acids, known as the building blocks of life, combine in different sequences to create proteins, which are responsible for the vast majority of biological functions. The research team discovered that by selecting specific amino acids for their molecules, they could guide the self-assembly process to produce a variety of materials, from soft, gel-like substances to much harder, spherical forms—akin to Maltesers. This controlled assembly opens new doors for a range of possible applications that could have significant impacts in fields ranging from photonics to biomedical engineering.
As the team delved deeper into the chemistry of these molecules, they found that adding external elements, like lanthanide ions, further enhanced their utility. Notably, these modified “Malteser-like” molecules began to exhibit luminescent properties—key to their potential in optical applications, such as sensors and biomedical tools that monitor molecular interactions within living systems.
Reproducing Nature’s Precision: The Power of Controlled Self-Assembly
The critical breakthrough of the research team was not only in producing these functionalized molecules but also in their ability to achieve consistent and predictable outcomes through controlled self-assembly. Until now, the precise mechanisms that govern how molecules self-assemble in biology have been largely a mystery. Many natural processes, like those used by proteins to fold into specific structures, occur with an almost astonishing level of precision that makes the molecular machinery of life so reliable. Researchers have strived to understand and mimic this self-assembly at the molecular level in synthetic systems for years, but the complexity of doing so in a repeatable way has been a daunting challenge.
Through the development of their amino acid-based ligands, the team found a method to create structures that are consistent and repeatable. By simply altering the amino acid components and the presence of additional elements, they were able to engineer molecules that self-assemble into specific, predictable configurations. This opens up the possibility of designing molecules that perform predetermined functions, such as capturing and releasing drugs in a highly targeted manner, or even creating advanced sensors capable of detecting specific biological markers.
This breakthrough could have profound implications in medicine, allowing for the development of advanced targeted therapies. For example, drugs could be designed to remain inert in the body until they encounter specific enzymes—enzymes that are upregulated in response to certain conditions, like infections. Once these enzymes break down the molecules, the drug would be released at the site where it’s needed most, minimizing the side effects commonly associated with less targeted treatments.
Additionally, the ability to monitor molecular interactions in real time—thanks to the luminescent properties of the molecules—could allow for unprecedented insights into biological processes. This level of monitoring could lead to better diagnostics and a deeper understanding of how diseases progress within the body.
Luminescent Molecules and Real-Time Monitoring
Another key feature of the self-assembled molecules developed in this study is their ability to emit circularly polarized light when functionalized with lanthanide ions. This property makes them suitable for applications in both biomedical imaging and optoelectronics. Luminescent molecules are already used in a variety of imaging technologies, but the unique emission properties of these new molecules could significantly enhance our ability to track and visualize biological interactions in living organisms.
Dr. Oxana Kotova, a member of the TBSI team, highlighted the importance of the luminescent nature of these molecules. In collaboration with Professor Robert Pal at Durham University, Kotova and her colleagues discovered that the light emitted by their engineered molecules is not only bright but also circularly polarized. This characteristic makes it ideal for tracking specific interactions within biological media, such as when certain proteins bind to targeted molecules, or when drugs are released within cells. The precision of circularly polarized light could allow for sharper, more detailed imaging in biological settings.
Prof. Gunnlaugsson further elaborated on the potential applications for these “Malteser-like” molecules in real-world technologies. The ability to achieve precise molecular self-assembly on command means these molecules could one day find practical applications in photonic devices, optical systems, or the burgeoning field of nanoscale sensors. As sensors in biomedical devices, they could detect a wide range of chemical signals, providing real-time diagnostics with a level of sensitivity that could radically improve health outcomes.
Moving Forward: Challenges and Opportunities
The work presented by the researchers in this study represents an exciting leap forward in molecular engineering and chemistry. However, as Prof. Gunnlaugsson emphasized, there is still much more to learn. The study reveals just the tip of the iceberg, as researchers continue to explore new ways of programming molecules to self-assemble for specific applications. The potential for these molecular systems is vast, but their engineering requires much further exploration and refinement.
In this process, it is crucial for the scientific community to learn from the natural world. Nature has spent billions of years refining the molecular processes that allow life to function with such complexity and precision. Researchers are now trying to replicate these feats, not only by mimicking nature’s processes but by refining them with human ingenuity. As scientists improve their understanding of self-assembly, they will continue to unlock new possibilities, turning molecular processes into the basis for practical, impactful innovations in science and medicine.
Implications for the Future
The research on self-assembling molecules has the potential to spark innovations across multiple fields, particularly in medicine and advanced materials. Molecular systems that can self-assemble on command could change the way we design drug delivery systems, allowing for treatments that target specific sites in the body while minimizing side effects. Additionally, these molecules could be used to create advanced materials with properties that are hard to achieve through traditional manufacturing methods. As these breakthroughs continue, self-assembly could also play a role in nanotechnology, enabling the construction of highly specialized nanoscale structures with applications in everything from electronics to materials science.
Ultimately, as this research matures, we could find ourselves on the precipice of a new era in biotechnology, one in which we can design molecules not just to survive and function, but to work with precision and purpose—bringing us one step closer to controlling molecular processes with the same elegance that nature has honed over millennia.
Reference: Aramballi J. Savyasachi et al, Exerting control of self-assembly pathways via morphological switching and patterning in amino-acid-based benzene-1,3,5-tricarboxamide conjugates, Chem (2024). DOI: 10.1016/j.chempr.2024.09.020
Think this is important? Spread the knowledge! Share now.