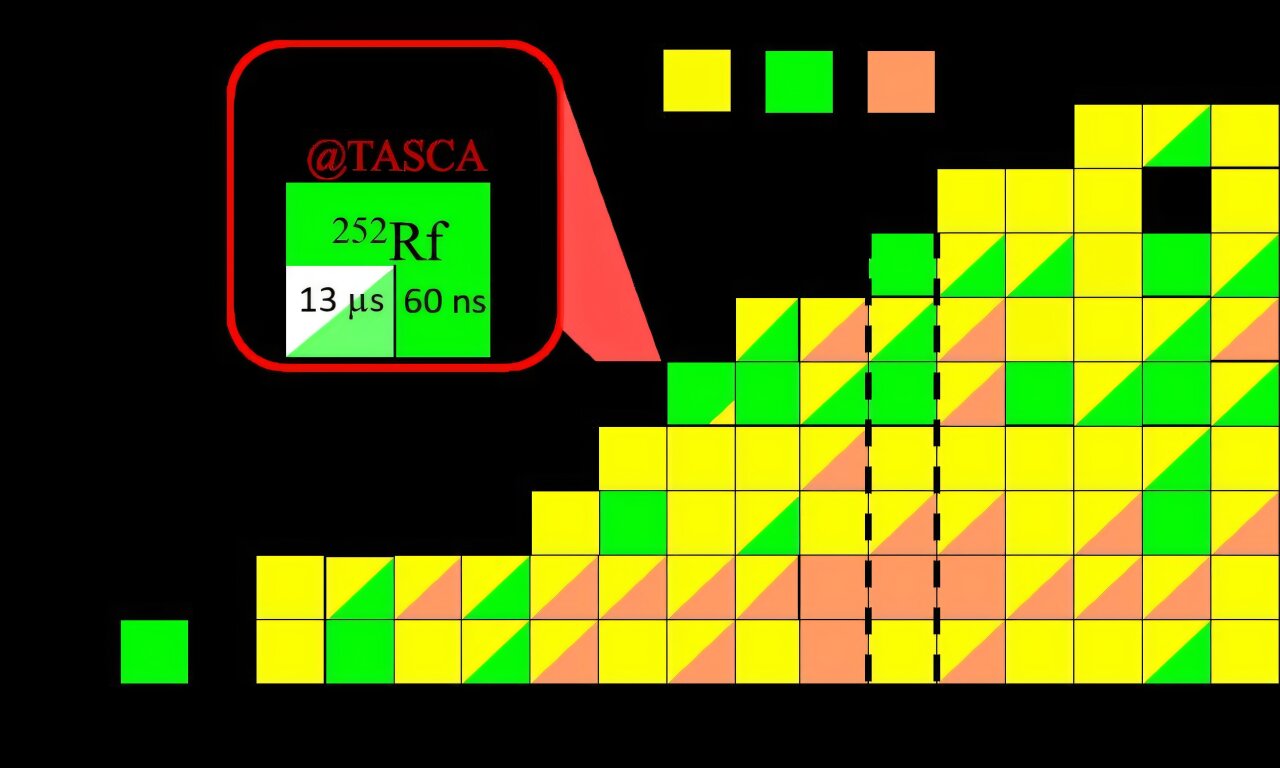
A groundbreaking new study, published in Physical Review Letters, reveals a significant step forward in the quest to explore the “island of stability” within the realm of superheavy nuclei. A team of researchers from GSI/FAIR, Johannes Gutenberg University Mainz, and the Helmholtz Institute Mainz have succeeded in more precisely exploring the limits of this hypothesized region by studying the recently discovered, and shortest-lived, superheavy nucleus, Rutherfordium-252 (Rf-252). This discovery marks a vital advancement in understanding the long-standing theoretical predictions for superheavy elements.
The Island of Stability: What Lies Beyond the Sea of Instability?
Atomic nuclei are made up of protons and neutrons, which interact via the strong force that holds them together. This force is key to maintaining the integrity of the nucleus despite the repulsion between the positively charged protons. However, the more protons a nucleus has, the greater this repulsive force becomes, which can lead to nuclear instability. This is a significant obstacle for researchers trying to create and explore new superheavy elements, which are nuclei with many protons and neutrons.
The concept of the island of stability emerged in the 1960s, with theoretical predictions suggesting that there are certain “magic numbers” of protons and neutrons that create particularly stable configurations of matter within heavy nuclei. These magic numbers correspond to numbers of protons or neutrons that form full shells, similar to how noble gases are chemically stable because of their full electron shells.
For superheavy elements, researchers have predicted that a specific combination of protons and neutrons could lead to long-lived nuclei. This region, where nuclei exhibit significantly longer lifetimes, is referred to as the island of stability. Notably, such long-lived nuclei could theoretically persist for millions, or even billions, of years, approaching the age of the Earth itself. However, the exact location, size, and half-life of this island have remained elusive, largely due to the inherent challenges of creating these superheavy nuclei and detecting their elusive lifetimes.
Discovery of Rutherfordium-252 and Its Significance
Rutherfordium (Rf), with an atomic number of 104, is one of the heavy elements at the outer boundaries of the periodic table. The new discovery—Rf-252—is of particular interest because it is now the shortest-lived superheavy nucleus known, with a half-life of only 60 nanoseconds, a remarkable but fleeting moment in time. While such a short-lived nucleus would normally elude detection due to the limits of available measurement techniques, the researchers were able to observe it using clever methods that make it possible to study even these extremely unstable nuclei.
To detect Rf-252, the researchers needed a precise technique, as traditional methods would fail for nuclei with half-lives on the order of a millionth of a second. The team turned to quantum effects to identify excited states in the nucleus that could have longer lifetimes than the ground state. These excited states, known as isomers, are akin to “stable clouds” above the “sea of instability” of nuclei. They are crucial to advancing the picture of the island of stability by providing more accessible points to measure.
Dr. Khuyagbaatar Jadambaa, a leading researcher on the study from GSI/FAIR, elaborated on the significance of excited states: “Such long-lived excited states, so-called isomers, are widespread in superheavy nuclei of deformed shape, according to my calculations. Thus, they enrich the picture of the island of stability with ‘clouds of stability’ hovering over the sea of instability.” Isomers, stabilized by these quantum effects, allow researchers to probe superheavy elements that otherwise decay too rapidly for direct measurement.
By synthesizing the Rf-252 nucleus, the team was able to take an essential step in advancing our understanding of the boundary region of superheavy elements where these nuclei begin to transition from the chaotic instability of lighter elements into a regime of greater stability. This region is the hypothesized “shoreline” of the island of stability, and the discovery of Rf-252 has confirmed that this boundary exists in the range of atomic number 104.
A Journey from Experiment to Discovery
The experimental approach was centered on the powerful UNILAC (Universal Linear Accelerator) at GSI/FAIR, where the researchers used a titanium-50 beam to fuse it with a lead target foil. These energetic collisions produced various isotopes, including the previously undiscovered Rf-252. Using the TransActinide Separator and Chemistry Apparatus (TASCA), the products of these collisions were carefully separated and implanted into silicon detectors. This allowed scientists to track the resulting decay patterns and confirm the existence of Rf-252.
In total, the team observed 27 atoms of Rf-252, each undergoing fission after decaying for an astonishingly brief 60-nanosecond half-life. Through the study of the decays and emissions, especially in relation to excited states of Rf-252, the team was able to discern that the actual half-life for the ground state of the nucleus was 60 nanoseconds, currently the shortest-lived superheavy nucleus known.
“This result decreases the lower limit of the known lifetimes of the heaviest nuclei by almost two orders of magnitude,” said Professor Christoph E. Düllmann, head of the Superheavy Element Chemistry Department at GSI/FAIR. “It sets a new benchmark for future studies related to fission stability, isotopic boundaries in superheavy elements, and even the possibility of nuclei where excited states may hold greater stability than ground states.” This discovery may also play a central role in the upcoming study of next heavier elements, particularly seaborgium (Sg, element 106).
What’s Next? Expanding the Frontiers of Superheavy Nuclei
The precise mapping of the shoreline of the island of stability offers several exciting avenues for future research. One of the primary focuses of the team’s next steps will be the exploration of inverted fission stability, where certain excited nuclear states exhibit more stability than the nucleus’s ground state. These states will be particularly important as experiments on seaborgium isotopes, with half-lives even shorter than those of Rf-252, are pursued in future experimental campaigns.
The continuing work on mapping these borders with superheavy nuclei, especially within the framework of new international collaborations such as the construction of the FAIR facility (Facility for Antiproton and Ion Research) in Darmstadt, Germany, could expand the scope of what is currently known about the island of stability. A key target will be isotopes of seaborgium (Sg) with incredibly short-lived nuclei, as well as the study of new isomer states.
The findings related to the discovery of Rf-252, and particularly its detailed investigation, are expected to significantly impact the ongoing effort to push the boundaries of the periodic table. This cutting-edge research opens up new possibilities not only in the understanding of nuclear physics but also in potentially harnessing superheavy elements for future applications in various scientific and technological realms.
Conclusion
The study of superheavy elements at the edge of the periodic table is rapidly evolving, and the detection of Rutherfordium-252, with its incredibly short-lived nature, represents a remarkable step forward in the understanding of the island of stability. By advancing our ability to observe and measure such fleeting, heavy nuclei, this research is laying the groundwork for mapping out the limits of matter’s stability at the most extreme ends of the periodic table.
As scientists continue to push forward with high-energy experiments at the state-of-the-art facilities like GSI/FAIR and future developments at the FAIR accelerator, the island of stability might one day reveal its true potential, offering up new discoveries that will challenge our understanding of the very fabric of the universe.
Reference: J. Khuyagbaatar et al, Stepping into the Sea of Instability: The New Sub- μs Superheavy Nucleus Rf252, Physical Review Letters (2025). DOI: 10.1103/PhysRevLett.134.022501