A groundbreaking study from the Karolinska Institutet has provided unprecedented insights into the cellular and molecular mechanisms underlying human wound healing. Published in Cell Stem Cell, the research offers a detailed map of how various cells and molecules interact during different phases of the wound-healing process, shedding light on potential reasons why some wounds heal poorly. This work not only deepens our understanding of the biology of healing but also lays the foundation for innovative therapeutic strategies to address chronic wounds.
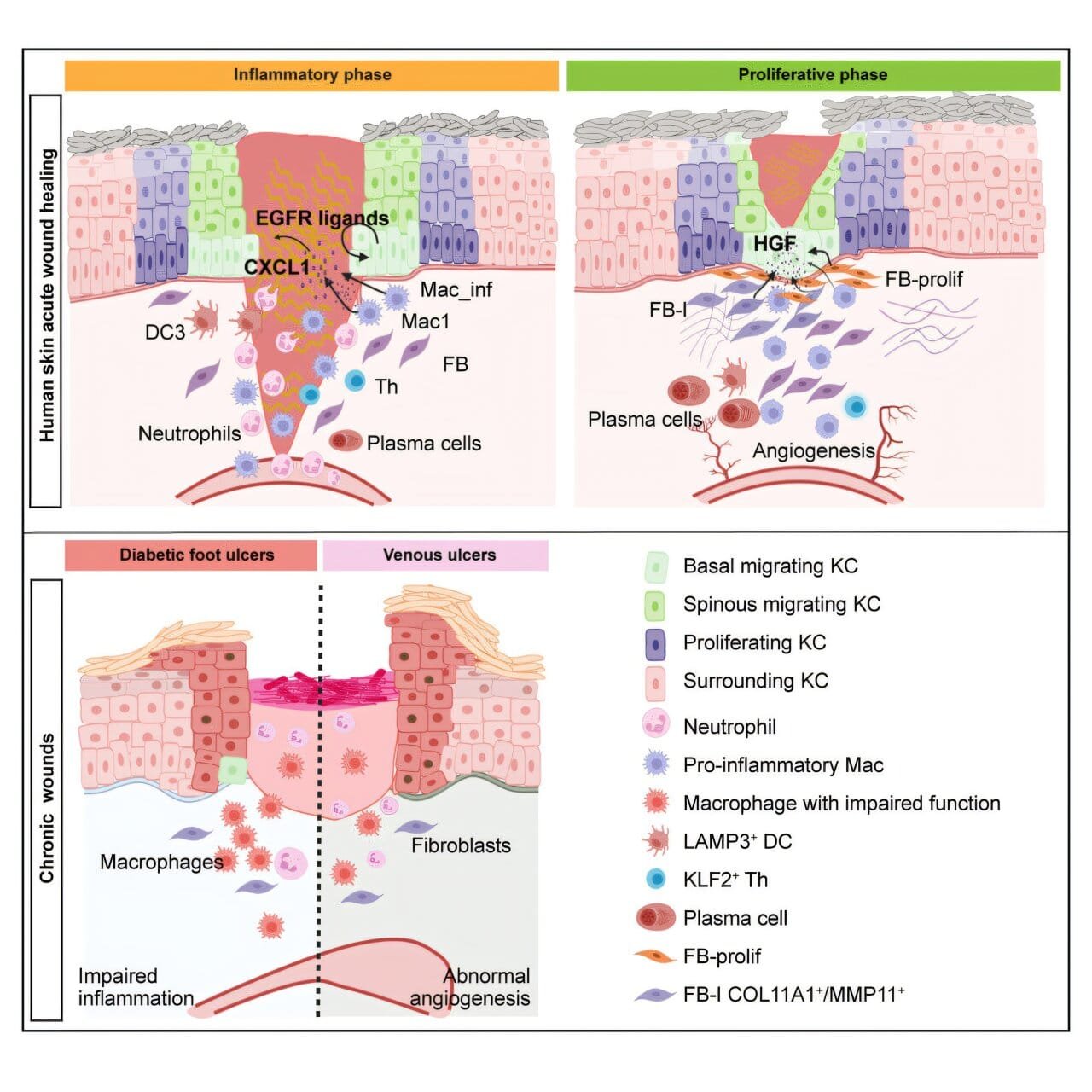
Wound healing is a complex, multi-phase process essential for human survival. Despite its importance, much remains unknown about how different cell types coordinate and communicate to repair damaged tissue. To bridge this knowledge gap, researchers at the Karolinska Institutet studied human skin and wounds from the same individuals, tracking changes across three critical phases of healing: inflammation, proliferation, and remodeling. These phases represent the body’s systematic approach to closing wounds and restoring normal function.
The team employed advanced techniques such as single-cell RNA sequencing and spatial transcriptomics. These state-of-the-art methods enabled them to map the activity of individual cells and the spatial arrangement of molecular signals over time. The resulting data revealed a dynamic interplay of cellular behaviors and molecular signals, providing a high-resolution view of the wound-healing process.
One key discovery involved the protein FOSL1, which plays a crucial role in enabling skin cells to migrate and cover wounds. “We have discovered that an important protein, FOSL1, helps skin cells to move and cover wounds during the healing process,” explains Zhuang Liu, a postdoctoral researcher in the Department of Medicine, Solna, at Karolinska Institutet. This finding highlights the molecular mechanisms that drive the movement of epithelial cells, a critical step in wound closure.
Additionally, the study emphasized the supportive roles of other cell types, such as macrophages and fibroblasts. Macrophages are immune cells that manage inflammation and clear debris, while fibroblasts are connective tissue cells that produce collagen and extracellular matrix components, forming the scaffold for new tissue. Together, these cells create an environment conducive to repair. “We have also seen that certain other cells, such as macrophages and fibroblasts, help these skin cells to move and repair the damage,” Zhuang adds.
The research also explored why chronic wounds, such as venous ulcers and diabetic foot ulcers, often fail to heal properly. By comparing chronic wounds to acute wounds, the researchers identified key differences in inflammatory responses and cellular migration. Chronic wounds showed impaired cell movement and persistent inflammation, which hinder the healing process. “When we compared wounds from people with chronic diseases, such as venous ulcers and diabetic foot ulcers, we found that problems with cell movement can make healing more difficult,” says Zhuang.
These findings are especially significant in light of the global burden of chronic wounds, which affect millions of people and are often associated with conditions like diabetes, vascular diseases, and aging. Chronic wounds not only reduce quality of life but also impose substantial healthcare costs. Understanding the molecular and cellular barriers to healing in these cases could lead to targeted therapies that restore normal function and improve outcomes for patients.
Importantly, the study also highlighted the unique characteristics of human wound healing compared to animal models. Most previous research has relied on animal studies, which, while valuable, do not fully replicate the complexities of human biology. “Our findings also underscore the unique characteristics of human skin wound healing, which differ significantly from animal models,” notes Ning Xu Landén, an associate professor at the Department of Medicine, Solna. This distinction is critical for translating basic scientific discoveries into effective clinical interventions.
The implications of this study are vast. By providing a detailed map of the cellular and molecular dynamics of wound healing, it opens the door to new therapeutic approaches. For instance, targeting FOSL1 or enhancing the activity of supportive cells like macrophages and fibroblasts could accelerate wound closure. Similarly, modulating inflammatory responses in chronic wounds could help resolve persistent healing barriers.
Reference: Zhuang Liu et al, Spatiotemporal single-cell roadmap of human skin wound healing, Cell Stem Cell (2024). DOI: 10.1016/j.stem.2024.11.013