Girolline, a natural compound extracted from the sea sponge Pseudaxinyssa cantharella, has recently gained attention for its potential medicinal properties. Initially, it was investigated for its antitumor effects, with some early studies suggesting it could serve as an effective treatment for cancer. More recently, researchers have discovered that girolline also has anti-malarial properties, further enhancing its appeal as a therapeutic agent. New findings from scientists at the RIKEN Center for Sustainable Resource Science have provided deeper insights into how girolline works, revealing new possibilities for its use, including as a chemical probe for studying aging, mitochondrial health, and other cellular functions.
Sea Sponges: Sources of Valuable Bioactive Compounds
The discovery of girolline highlights the significant role that marine organisms, particularly sea sponges, play in providing bioactive compounds that can have profound biological effects. Sea sponges, unlike many other organisms, do not have the ability to move or seek shelter in the environment, which makes them highly dependent on chemical defenses for survival. Over time, these creatures have evolved a wide range of chemical compounds to protect themselves from predators, microorganisms, and environmental stressors. Girolline is one such compound that has been isolated from Pseudaxinyssa cantharella, a species of marine sponge. It is one of several biologically active molecules found in sea sponges, making these creatures an important source of natural products for pharmaceutical research.
Early Studies: Antitumor Effects and Mechanisms
The initial focus on girolline’s potential as an antitumor agent stemmed from its ability to inhibit translation—the process by which messenger RNA (mRNA) is converted into proteins by ribosomes in cells. Translation is a fundamental biological process, and any disruption in this process can have significant consequences for cell function. It was hypothesized that girolline’s antitumor effect might be due to its general ability to inhibit translation, thus preventing the production of proteins necessary for the growth and proliferation of cancer cells.
However, this idea was based on limited evidence, and there was little follow-up research into the detailed mechanisms by which girolline exerts its effects. While the idea of girolline being a “general translation inhibitor” seemed plausible, it left many questions unanswered about how the compound interacted with the translation machinery and whether its effects were selective or more widespread.
New Insights: Girolline’s Mechanism of Action
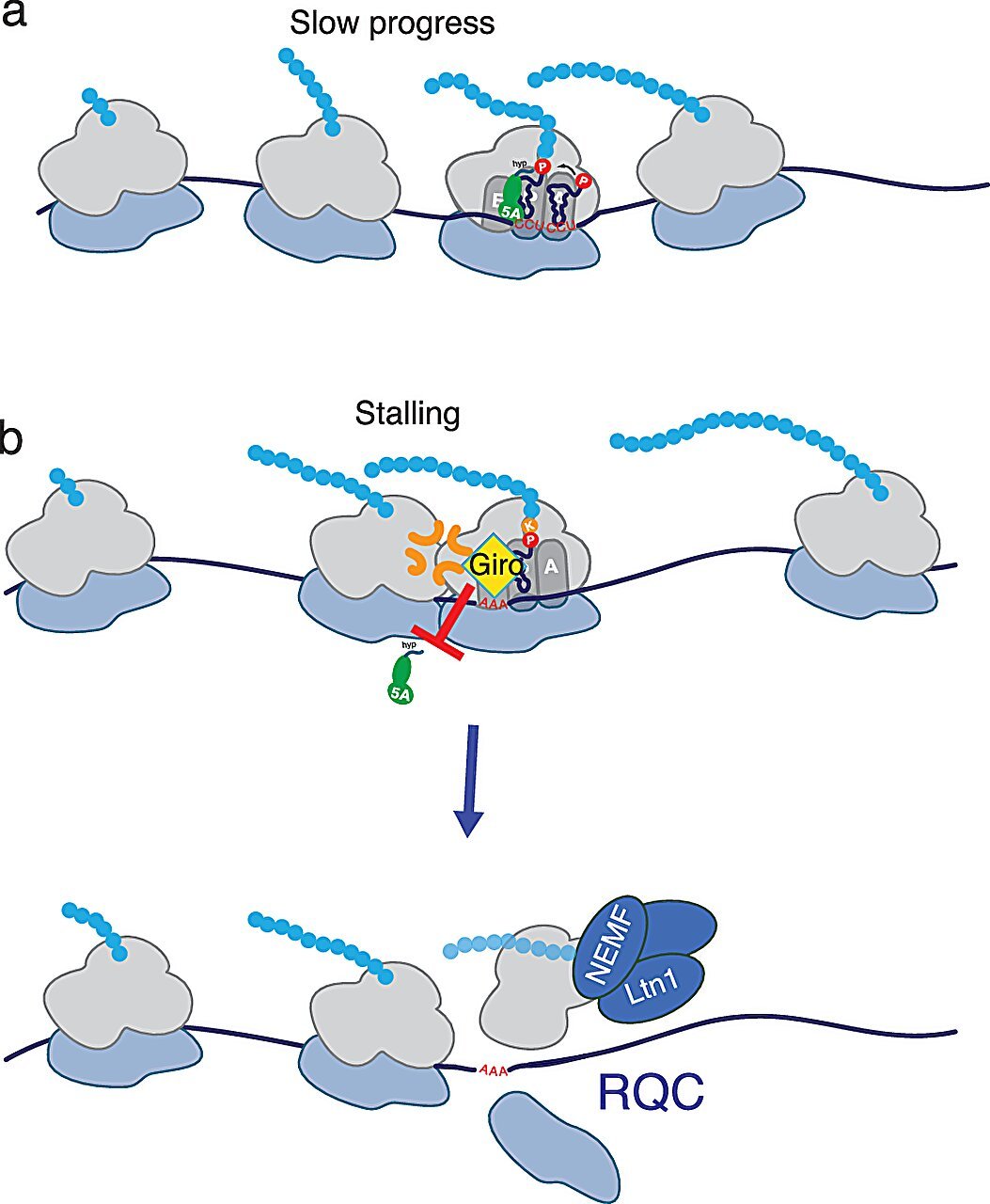
In a recent breakthrough study published in Nature Communications, scientists from the RIKEN Center for Sustainable Resource Science have uncovered a more precise and nuanced understanding of how girolline works. The research, led by Tilman Schneider-Poetsch, revealed that girolline does not inhibit translation indiscriminately but rather modulates the function of a specific protein synthesis factor called eIF5A (eukaryotic translation initiation factor 5A). This is a significant finding because, until now, no small molecule had been found to directly affect eIF5A in this manner.
The Role of eIF5A in Protein Synthesis
eIF5A is an important translation elongation factor that helps the ribosome navigate through challenging stretches of the mRNA sequence that are difficult to translate. These difficult stretches, often characterized by repetitive sequences of specific amino acids, can cause the ribosome to stall during protein synthesis. eIF5A works by binding to the ribosome and assisting in the incorporation of amino acids into the growing protein chain, thus ensuring that translation continues smoothly.
Girolline works by preventing eIF5A from binding to the ribosome. Without eIF5A’s assistance, the ribosome stalls when it encounters these difficult sequences, which include stretches of amino acids such as proline and lysine. Particularly problematic is the RNA sequence AAA, which encodes the amino acid lysine. When the ribosome stalls on these sequences, the ribosome-associated quality control (RQC) pathway is triggered, leading to the degradation of the unfinished protein.
This premature degradation of incomplete proteins is thought to contribute to girolline’s toxicity, as it disrupts the normal process of protein synthesis and leads to the accumulation of incomplete or misfolded proteins. While this mechanism helps explain girolline’s potential as an antitumor agent, it also provides valuable insights into the compound’s broader biological effects.
Implications for Malaria Treatment
One of the more exciting applications of girolline’s mechanism of action is its potential as a treatment for malaria. The malaria parasite Plasmodium falciparum is responsible for the most severe form of malaria, and its life cycle involves the production of many proteins that are synthesized in a manner similar to that of human cells. Messenger RNA (mRNA) molecules in Plasmodium often contain stretches with high numbers of adenine bases (the nucleotide “A”), which make them particularly susceptible to the effects of girolline. The adenine-rich sequences in Plasmodium falciparum’s mRNA are prone to causing ribosomal stalling, just as they do in human cells.
By inhibiting eIF5A and causing ribosomal stalling in Plasmodium cells, girolline could disrupt protein synthesis in the malaria parasite, leading to the degradation of essential proteins and ultimately inhibiting the parasite’s ability to survive and reproduce. This finding paves the way for further research into girolline as a potential anti-malarial treatment, which could be especially valuable in combating drug-resistant strains of Plasmodium.
Girolline as a Tool for Studying Aging and Mitochondrial Health
Beyond its potential as a therapeutic agent for cancer and malaria, girolline also holds promise as a valuable tool for studying fundamental biological processes such as aging and mitochondrial health. eIF5A has been implicated in the maintenance of mitochondrial function, which is critical for cellular energy production and overall cell health. Dysfunction in eIF5A has been linked to age-related diseases, neurodegeneration, and various cellular disorders.
By selectively modulating eIF5A’s activity, girolline could help researchers better understand how eIF5A contributes to mitochondrial maintenance and how its dysfunction contributes to the aging process. This makes girolline a potentially useful chemical probe for exploring the molecular mechanisms underlying aging and age-related diseases. Researchers are already exploring follow-up projects to investigate these aspects, hoping to gain new insights into how eIF5A functions in the context of mitochondrial health and aging.
Reference: Tilman Schneider-Poetsch et al, Girolline is a sequence context-selective modulator of eIF5A activity, Nature Communications (2025). DOI: 10.1038/s41467-024-54838-2