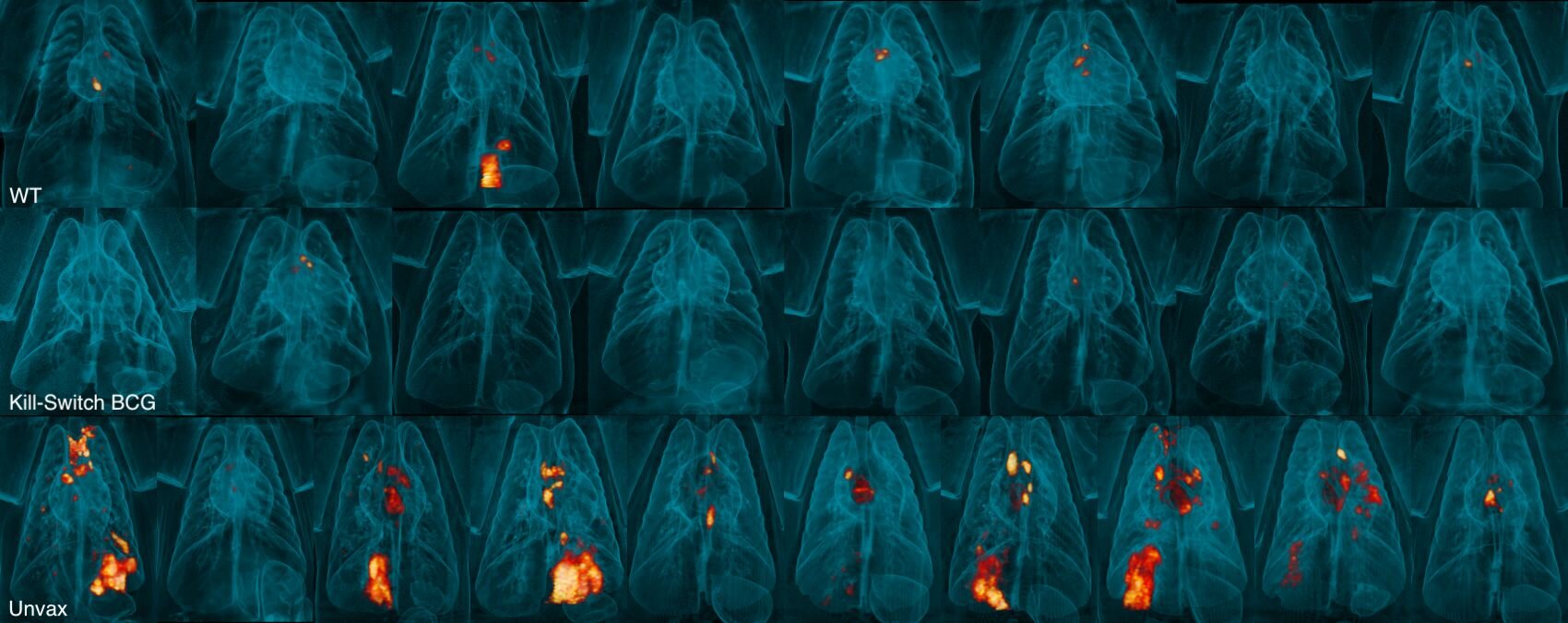
A novel approach to tuberculosis (TB) vaccination has shown promising results in research conducted by the University of Pittsburgh, suggesting an advanced method with an added layer of safety while delivering greater protection than the conventional approach. This innovation, involving a self-destructing vaccine administered intravenously, offers significant breakthroughs in combating TB in macaque monkeys, with potential implications for human health in the future. Published in Nature Microbiology, the study offers fresh hope for more effective and secure vaccination strategies in the global fight against tuberculosis, which the World Health Organization identified as the deadliest disease of 2024.
Tuberculosis continues to present a substantial global health challenge, with millions of people at risk of infection each year. However, existing protection methods—especially the widely-used Bacillus Calmette-Guérin (BCG) vaccine—are far from perfect. BCG is based on a strain of inactivated mycobacteria and has been the primary defense against TB for nearly a century. While BCG offers partial protection against childhood TB, it is largely ineffective in adults. Furthermore, its mode of administration via intradermal injections limits its overall efficacy and consistency, especially among vulnerable populations, such as immunocompromised individuals.
The new study, led by Dr. JoAnne Flynn, a distinguished professor at the University of Pittsburgh’s Department of Microbiology and Molecular Genetics, examines a more potent and safer alternative. Flynn’s team administered an intravenous (IV) version of the BCG vaccine, but with an ingenious twist. The vaccine was modified with a built-in self-destruction mechanism, allowing it to dissolve and “self-destruct” under controlled conditions, addressing the concerns associated with intravenous vaccination using live mycobacteria. This dual safety mechanism allows the vaccine to limit the risk of self-infection in case of accidental exposure.
“Although the idea of intravenous vaccination with a live vaccine may sound scary, it was very effective in our previous studies in non-human primates,” Dr. Flynn noted. “This time, we focused on improving safety by incorporating a kill-switch design in the vaccine strain, ensuring there would be no accidental long-term infection from the administration.”
This innovative safety mechanism works through a genetic “kill switch” built into the BCG vaccine strain, rendering it harmless and incapable of sustaining an infection. This switch is activated under two conditions: exposure to the antibiotic doxycycline or the cessation of doxycycline treatment. This safeguard eliminates the risk of the mycobacteria persisting in the body and causing infection, even in the case of an immunocompromised recipient.
To examine the effectiveness and safety of this modified IV vaccine, the researchers began with mouse models. Their results showed that the modified BCG vaccine, while similar to the traditional vaccine in its protective effects against TB, exhibited a more favorable safety profile. In these trials, the self-destructing BCG strain was rapidly cleared from the animals’ systems and showed no adverse effects, even when administered to immunocompromised mice. The effectiveness of this new approach was confirmed by the observed protection in the mice, comparable to the standard BCG vaccine.
Building on their encouraging results in mouse models, Dr. Flynn and her team tested the self-destructing vaccine in non-human primates—macaque monkeys—closely mimicking human TB infection. In these trials, the updated IV BCG vaccine proved not only safe but even more effective than the traditional IV BCG shot. Remarkably, none of the macaques that received the updated vaccine exhibited any detectable signs of lung inflammation, an indicator of infection or immune reaction. Eight weeks after exposure to live Mycobacterium tuberculosis, six out of eight monkeys that received the self-destructing vaccine had no recoverable traces of the bacteria in their lungs, compared to only two out of eight monkeys that received the standard BCG vaccine.
These findings provide significant hope for advancing TB vaccination efforts, as they indicate a more efficient and safer means of delivering the vaccine that could potentially provide sterilizing immunity—a term used when an organism’s immune system can clear an infection to the point where no traces of bacteria remain.
One of the most exciting features of the self-destructing BCG vaccine is its potential application in people with compromised immune systems. Traditional BCG vaccinations can be dangerous for immunocompromised individuals since there is a small risk that live bacteria may cause an active infection. However, the updated vaccine, with its built-in “kill switch,” provides a potential solution for a safer route of administration. In fact, one of the central motivations of this research was to create a vaccine that could benefit the immunocompromised population, such as those living with HIV or undergoing treatments like chemotherapy.
“By incorporating this self-destruction mechanism, we significantly mitigate the risk of vaccine-derived infection while providing an option for an effective vaccination route,” Dr. Flynn emphasized. “The updated vaccine gives us an exciting new approach in a situation where options for safe and effective vaccines are desperately needed.”
Nevertheless, there are challenges ahead. While these results are incredibly promising, they are still confined to animal trials, and the path to clinical testing in humans will be long and require thorough assessments for both safety and efficacy in human populations. The added complexities of regulatory approval, especially concerning a new mode of vaccination administration, may pose further obstacles. However, Flynn and her collaborators remain optimistic about the possibility of this novel vaccination approach reaching human trials and ultimately contributing to the global fight against TB.
“This research is an important step toward addressing TB in both humans and animals,” Dr. Flynn remarked. “We believe this new model will open the door to more effective TB vaccines and offer hope for those suffering from TB, including the immunocompromised, who are often most vulnerable.”
In the context of a rapidly changing world where emerging infectious diseases and antimicrobial resistance threaten public health, this self-destructing TB vaccine offers an exciting possibility in combating one of the oldest and deadliest diseases known to humankind. By advancing the safety of live vaccines and offering enhanced protection, it could become a critical tool in overcoming the global TB crisis. As research continues to evolve, innovations such as this one pave the way for more effective and accessible medical interventions, contributing not only to the control of tuberculosis but also to the broader mission of improving global health security.
Reference: A BCG kill switch strain protects against Mycobacterium tuberculosis in mice and non-human primates with improved safety and immunogenicity’, Nature Microbiology (2025). DOI: 10.1038/s41564-024-01895-4