Lithium-air (Li-O2) batteries have long been touted as a game-changer in energy storage, offering the potential to store much more energy than conventional lithium-ion (Li-ion) batteries while maintaining the same weight. This could pave the way for significant advancements in applications that demand high energy density, such as electric vehicles (EVs) and large-scale renewable energy storage systems. However, despite their promising theoretical advantages, lithium-air batteries have faced significant challenges in practical implementation. One of the biggest obstacles has been their short lifespan, stemming from issues such as sluggish electrochemical reactions, electrode degradation, and undesirable side reactions.
A new study published in Angewandte Chemie International Edition by a team of researchers from the Dalian Institute of Chemical Physics, part of the Chinese Academy of Sciences (CAS), proposes a novel solution that could overcome some of these barriers. By introducing a soluble catalyst to the electrolyte, the team aims to enhance the battery’s performance and extend its lifespan. The catalyst, a type of redox mediator, facilitates charge transport and helps prevent electrode passivation — one of the primary causes of capacity fade in lithium-air batteries.
The Promise and Challenges of Lithium-Air Batteries
Lithium-air batteries differ fundamentally from lithium-ion batteries in their design and operation. While lithium-ion batteries rely on the movement of lithium ions between two electrodes — typically a positive cathode and a negative anode — lithium-air batteries utilize an anode made of metallic lithium. As the battery discharges, lithium ions are released from the anode and migrate through the electrolyte to the porous cathode, where they interact with oxygen from the air. The oxygen molecules are reduced, forming lithium peroxide (Li2O2), a compound that stores the energy.
Upon charging, the lithium peroxide decomposes, releasing oxygen and allowing lithium ions to return to the anode, where they are reduced back to metallic lithium. This process theoretically enables lithium-air batteries to store far more energy than lithium-ion batteries because the cathode is essentially using the abundant oxygen from the air as a reactive component, rather than relying on the limited materials used in traditional batteries.
However, the theoretical high performance of lithium-air batteries has not translated into practical success. One of the primary issues is the overpotential, which refers to the excess voltage required to drive electrochemical reactions. The formation and decomposition of lithium peroxide (Li2O2) are inherently slow, and the solid Li2O2 is poorly conductive. Moreover, as the reactions proceed, the cathode’s pores can become clogged with the byproducts, hindering the efficient flow of oxygen. Furthermore, the high potential needed to form oxygen at the cathode often causes undesirable side reactions that degrade the electrolyte, contributing to a rapid decline in battery performance after only a few charge/discharge cycles.
A Novel Approach: Introducing a Soluble Catalyst
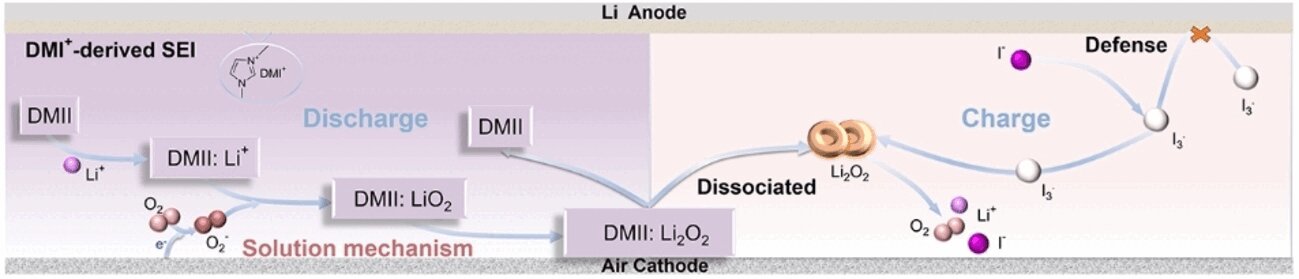
In their study, the team, led by Zhong-Shuai Wu from the Dalian Institute of Chemical Physics, collaborated with Xiangkun Ma from Dalian Maritime University to explore the potential of adding a soluble catalyst to the electrolyte. The catalyst, a type of imidazole iodide salt known as 1,3-dimethylimidazolium iodide (DMII), acts as a redox mediator. This substance has the ability to shuttle electrons between the oxygen molecules in the cathode and the lithium ions in the anode, thereby improving the electrochemical reactions that occur during discharge and charge cycles.
The iodide ions (I−) in the salt react reversibly to form I3−, a redox pair capable of transferring electrons between the lithium ions and the oxygen. When the battery is discharging, the I3− ions facilitate the transfer of electrons to the oxygen molecules at the cathode. During charging, the process reverses, with the iodide ions regaining their electrons, ready to participate in the next discharge cycle. This process effectively accelerates the reaction rates, reducing the overpotential associated with the cathode and enhancing the battery’s performance.
Mechanisms Behind the Improvement
The team discovered that the 1,3-dimethylimidazolium (DMI+) ions in the catalyst play a crucial role in improving battery performance. The DMI+ ions contain a heterocyclic ring composed of three carbon atoms and two nitrogen atoms, and these rings are rich in freely mobile electrons. These electrons enable the DMI+ ions to interact with and “capture” lithium ions during discharge, facilitating their transfer to the oxygen at the cathode. This ensures a more efficient discharge process, improving the overall performance of the battery.
In addition to improving the electrochemical reactions, the DMI+ ions help stabilize the anode. The DMI+ ions form an ultrathin but stable interface on the anode’s surface, preventing direct contact between the lithium metal and the electrolyte. This protective interface minimizes the decomposition of the electrolyte and prevents the formation of dendrites — spiny, metallic structures that can form on the anode and cause short circuits, further reducing battery lifespan. By stabilizing the anode, this interface extends the overall cycle life of the battery.
Promising Results from Electrochemical Tests
To test the effectiveness of their proposed solution, the researchers constructed electrochemical cells with the novel catalyst. The results were highly promising. The addition of DMII to the electrolyte led to several improvements over traditional lithium-air batteries:
- Reduced Overpotential: The battery demonstrated a very low overpotential of just 0.52 V, much lower than that typically observed in lithium-air batteries. This reduction in overpotential means that the battery can deliver higher performance without the need for excessive voltage, which in turn reduces energy losses during operation.
- Improved Cycle Stability: The team found that their modified cells exhibited exceptional cycle stability, maintaining their performance over 960 hours of charge/discharge cycles. This is a significant improvement over traditional lithium-air batteries, which typically degrade rapidly after only a few cycles.
- Highly Reversible Formation and Decomposition of Li2O2: One of the key challenges in lithium-air batteries is ensuring that the formation and decomposition of lithium peroxide (Li2O2) is highly reversible. The team’s cells demonstrated this property without any unwanted side reactions, which is a major step toward making lithium-air batteries more practical for long-term use.
Looking Ahead: The Future of Lithium-Air Batteries
This research marks a significant step forward in addressing the longstanding challenges faced by lithium-air batteries. By introducing a soluble catalyst to the electrolyte, the team has enhanced the electrochemical performance and stability of these batteries, bringing them closer to realizing their theoretical potential. The reduction of overpotential, increased cycle stability, and improved efficiency of the Li2O2 formation/decomposition cycles could pave the way for lithium-air batteries to replace conventional lithium-ion batteries in high-demand applications.
While there are still challenges to be overcome, such as scaling up this approach for practical, commercial use and ensuring that the electrolyte remains stable over extended periods, the findings from this study open up new possibilities for the development of high-performance energy storage systems. In particular, the approach could be of great significance for electric vehicles and renewable energy storage, where the need for high-capacity, long-lifetime batteries is critical.
As researchers continue to refine lithium-air battery technology, innovations like this could help usher in a new era of energy storage that is both more efficient and more sustainable, ultimately accelerating the transition to cleaner energy sources and reducing the environmental impact of energy storage systems.
In conclusion, while lithium-air batteries have faced significant hurdles in the past, the integration of redox mediators like DMII may be the key to unlocking their full potential. With further research and development, these batteries could one day become a cornerstone of next-generation energy storage technologies.
Reference: Jing Liu et al, A Bifunctional Imidazolyl Iodide Mediator of Electrolyte Boosts Cathode Kinetics and Anode Stability Towards Low Overpotential and Long-Life Li-O2 Batteries, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202421107
Loved this? Help us spread the word and support independent science! Share now.