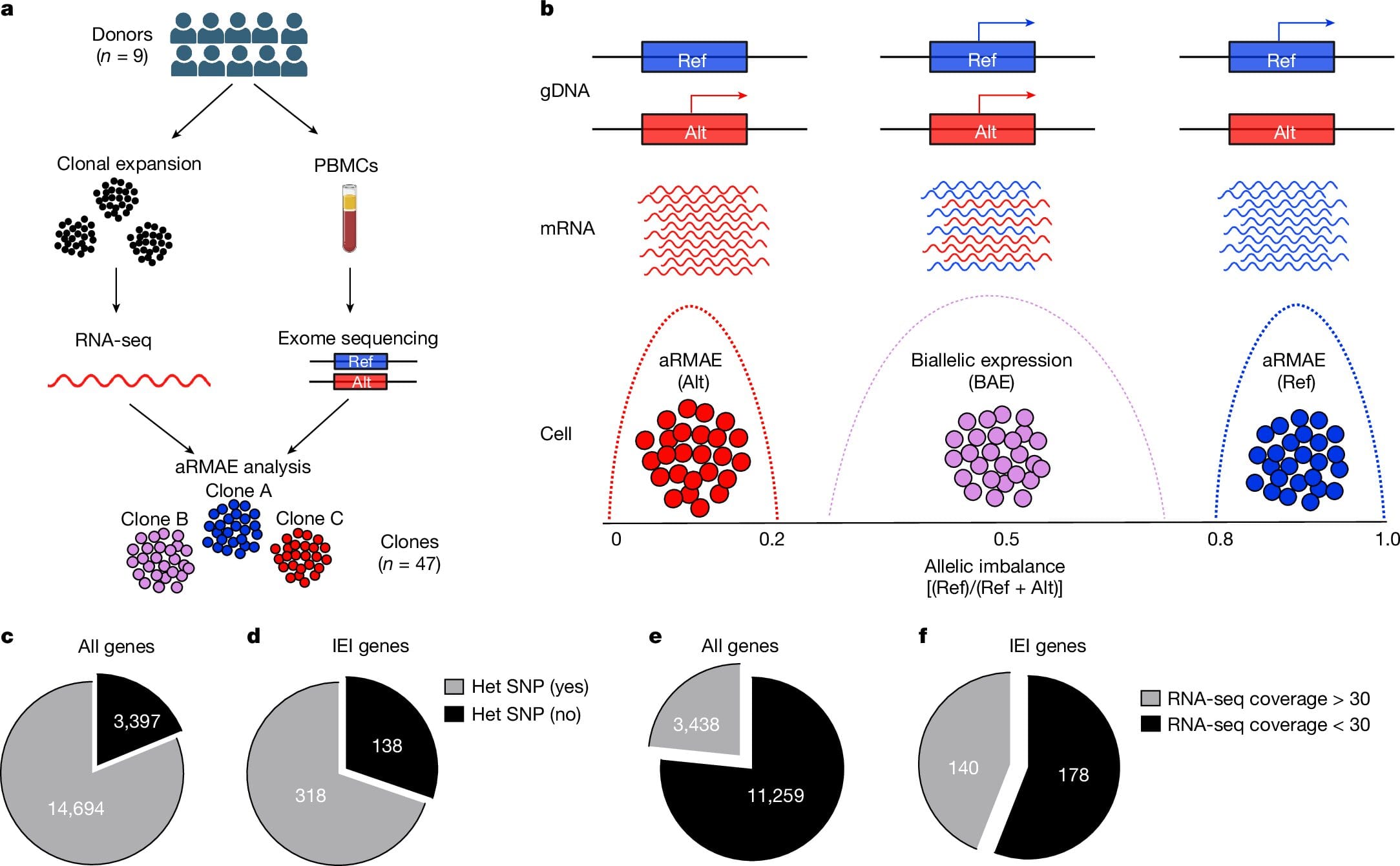
Genetics has long been an essential cornerstone of biology, deeply influencing our understanding of diseases and how traits are inherited. A common and fundamental principle taught to every biology student revolves around the concept of the genetic blueprint: each of our cells, except sperm and eggs, contains two copies of each gene, one inherited from the mother and the other from the father. For years, this principle suggested that the two gene copies played equal roles in our biological functioning. However, new groundbreaking research from Columbia University has unveiled an exciting and significant modification to this traditional genetic view.
This study, led by Dusan Bogunovic, a professor of pediatric immunology at Columbia University Vagelos College of Physicians and Surgeons, turns a well-established concept in genetics upside down. Bogunovic’s team has provided new insights into how our genetic material functions in a far more complex and nuanced manner than previously understood. They have uncovered an intriguing phenomenon where some of the cells in our bodies preferentially inactivate one of the two copies of a gene—the maternal or the paternal copy—and this selective bias might help explain the vast range of symptoms—or lack thereof—experienced by individuals who carry the same disease-causing genetic mutations. The study, published on January 1st, 2025, in Nature, presents a new view of our genetic activity that has significant implications for the study of genetic diseases.
This phenomenon, long speculated but never experimentally proven, adds a layer of complexity to our understanding of genetic diseases. In simplest terms, genetics involves two copies of every gene, one from the mother and one from the father. Until recently, the general understanding was that both copies should play an equal role in biological processes. However, researchers now reveal that, in reality, some cells demonstrate bias towards either the maternal or paternal gene copy, meaning that one parent’s gene might be preferentially active in certain cells. This surprising finding flips the long-accepted paradigm, shedding light on how genes are expressed in a way that varies across cells, tissues, and over time.
During the study, the researchers explored the immune cells of ordinary individuals to investigate how gene expression varies across different genetic lines. They discovered that, in a wide variety of cells, one of every twenty genes had undergone inactivation of one of the parental gene copies. For example, in some individuals, an immune cell might selectively shut down the maternal or paternal gene variant for specific genes. This finding could offer a major breakthrough in explaining the phenotypic variation, or differences in physical traits and symptoms, seen in various genetic diseases.
“This is suggesting that there is more plasticity in our DNA than we thought before,” says Dusan Bogunovic, explaining the newfound flexibility and complexity of genetic expression. His team’s discovery shows that, depending on the type of cell and potentially other factors like environmental influences or age, gene expression patterns can differ dramatically within the same individual. Some immune cells may express more of one parent’s gene, while kidney cells may be more influenced by the other parent’s copy. This new perspective opens doors to understanding why the same disease-causing mutations can have vastly different manifestations in individuals.
The research’s implications for genetic diseases are profound. In genetic medicine, certain mutations are associated with particular disorders, but it’s been long known that not everyone with a genetic mutation develops symptoms at the same rate or severity. For example, in several hereditary diseases, up to 90% of individuals who inherit a disease-causing mutation show symptoms, while others, who carry exactly the same mutation, remain completely symptom-free. This paradox of “genetic carriers” has puzzled scientists for years. Until now, the mechanism behind this inconsistency was not fully understood. But with the new discovery, researchers have presented the idea that this differential gene activity—whether due to biased gene silencing or selective gene inactivation—could be a fundamental contributor to these variations in disease severity.
Bogunovic and his team studied several families suffering from immune system disorders caused by inherited genetic mutations. Through this study, they observed that in the healthy individuals carrying the same mutations as their sick relatives, there was evidence that the disease-causing gene copy was more likely to remain inactivated or suppressed in immune cells. In contrast, the sick individuals showed an increased activity of the disease-related gene copy. This finding provides experimental evidence for the idea that the activation or suppression of gene copies, specifically the bias towards either the maternal or paternal gene variant, can influence whether a disease manifests and, if it does, how severe it might become.
While this finding was initially observed in immune system-related genetic disorders, the team believes that this selective inactivation is not limited to just one class of genes. According to Bogunovic, this phenomenon extends beyond immune system genes and could explain variability in a much wider range of genetic conditions. The broader impact could be significant in genetic disorders ranging from rare congenital diseases to more common conditions like cancer or autoimmune diseases.
The findings of the study could provide a critical new angle for understanding diseases that show flares or conditions that arise due to environmental factors, such as lupus or diseases influenced by stress, diet, or infections. The idea that gene expression varies between different individuals or even different cell types could help unravel the complex relationships between genetic predisposition and environmental influences, leading to a more nuanced understanding of how diseases progress or worsen over time.
What sets this study apart is the introduction of a new paradigm for diagnosing and treating genetic diseases. Historically, diagnosing genetic diseases has been largely focused on an individual’s genotype—that is, the specific mutations or genetic changes a person carries. However, as shown in this study, merely looking at a person’s DNA doesn’t always reveal the whole picture. Instead, researchers propose introducing the concept of the “transcriptome” in addition to the traditional “genotype.” The transcriptome refers to the total set of genes that are actively transcribed in a particular cell or tissue. Importantly, gene activity patterns (transcriptomes) can differ from one type of cell to another—and even change with time. With this new understanding, doctors could potentially include transcriptomic data in their diagnoses to better predict how genetic mutations will affect individual patients and how they might respond to treatment.
This approach not only adds more precision to genetic testing but also lays the foundation for potentially pioneering treatments. By understanding how selective gene inactivation occurs, it may become possible to devise new treatments that modify gene activity. For example, scientists could potentially “switch off” or “switch on” specific copies of a gene, effectively neutralizing a harmful mutation. Such strategies could lead to groundbreaking new ways to treat genetic diseases by adjusting patients’ gene expression patterns in a targeted way.
Although these therapeutic concepts are still a long way from being available in clinical practice, the fact that researchers can already manipulate gene expression in cell cultures is an encouraging first step. Bogunovic is optimistic that, one day, understanding the underlying mechanisms of gene expression in cells could lead to innovative therapies capable of converting a genetically disease-afflicted individual into one who is healthy. The potential for such transformative treatments could radically change the way genetic diseases are managed and cured in the future.
The study marks the beginning of a major shift in how scientists understand genetic expression, disease onset, and variability. It expands the landscape of genetic research beyond simply studying the DNA code and offers new avenues to personalize medicine based on how genes are actively expressed in cells. As more research in this area continues, the hope is that it will pave the way for advancements that bring both a deeper understanding and novel approaches to genetic diseases, leading to better diagnostic and treatment strategies tailored to the complexities of individual gene activity.
Reference: O’Jay Stewart et al, Monoallelic expression can govern penetrance of inborn errors of immunity, Nature (2025). DOI: 10.1038/s41586-024-08346-4