In the grand theater of chemistry, few rivalries are as ancient, fundamental, and captivating as that of acids and bases. They are the yin and yang of the chemical world, eternally balancing and opposing each other, shaping not only the reactions that fuel our bodies and the processes that sustain our planet, but also the very flavor of our foods and the effectiveness of our household cleaners.
Yet for all their importance, acids and bases remain a mystery to many. What exactly makes a substance an acid or a base? Why do lemons taste sour and soap feels slippery? How can a single drop of a solution change color so dramatically when mixed with another? And what deeper, hidden forces are at play behind these everyday experiences?
To understand acids and bases is to step into one of the most elegant and powerful frameworks science has ever created — a journey that leads from ancient alchemical notions to modern theories that touch the very structure of atoms and molecules.
Ancient Curiosity: The First Encounters
Long before chemistry was a formal science, early civilizations noticed that some substances had peculiar and consistent properties. Vinegar, extracted from spoiled wine, stung the tongue and preserved food. Mineral waters from certain springs had a bitter, slippery taste. Without microscopes or spectrometers, these early observers could only catalog what they saw and felt, but they were laying the groundwork for the acid-base story.
Ancient alchemists, fascinated by the transformative powers of nature, recognized that some substances corroded metals, others neutralized these corrosions, and still others could be combined to create new materials altogether. The words themselves hint at their historic roots: “acid” comes from the Latin acidus, meaning “sour,” while “alkali” (an old term for bases) derives from the Arabic al-qaly, referring to the ashes of plants used to make soap.
It would take centuries, however, before scientists could begin to explain these behaviors with any precision.
The Birth of Modern Understanding
The turning point came during the Age of Enlightenment, when thinkers like Robert Boyle, Antoine Lavoisier, and Svante Arrhenius began to strip away mysticism and look at substances through the lens of reproducible experiments and logical theory.
Boyle, in the 17th century, was one of the first to attempt a systematic study of acids and bases. He noted that acids were sour, reactive with metals, and changed the color of certain dyes — early indicators of their presence. Bases, on the other hand, were bitter, slippery to the touch, and could counteract acids.
Lavoisier, in the late 18th century, proposed that acids were substances containing oxygen. Though this hypothesis would later prove incomplete, it pushed scientists to look at the composition of materials rather than just their taste or feel.
The real leap came in the late 19th century when Svante Arrhenius proposed his groundbreaking definitions: acids were substances that increased the concentration of hydrogen ions (H⁺) in solution, and bases were substances that increased the concentration of hydroxide ions (OH⁻). This ionic theory finally connected the behavior of acids and bases to the structure of matter itself.
But even Arrhenius’ work was only part of the picture. As scientists explored reactions in non-water environments and discovered substances that acted like acids or bases without producing H⁺ or OH⁻, they had to expand their definitions. Thus emerged the Brønsted-Lowry theory (acids are proton donors, bases are proton acceptors) and the Lewis theory (acids accept electron pairs, bases donate electron pairs), each deepening our understanding and broadening the scope of acid-base chemistry.
The Essence of Acidity and Basicity
At its heart, the difference between an acid and a base boils down to the movement of tiny, fundamental particles — protons (hydrogen ions) and electrons. Acids, in their most primal form, are proton givers. They donate hydrogen ions, tiny positively charged packets of energy, to other molecules or ions. Bases, their eternal dance partners, are proton takers — either by snatching up hydrogen ions or by donating electrons that attract these protons.
This subtle shuffling of particles underpins the vivid, often violent chemistry that acids and bases can unleash. When an acid and a base meet, a spectacular transformation occurs: neutralization. The proton from the acid and the hydroxide ion from the base combine to form water, one of the simplest and most stable molecules in the universe. Along the way, energy is often released, salts are formed, and the very structure of the substances involved is altered.
It is a dance as old as the stars themselves.
pH: Measuring the Balance
In the 20th century, Danish chemist Søren Sørensen introduced a simple, elegant way to express the strength of acids and bases: the pH scale. Standing for “potential of hydrogen,” pH is a logarithmic scale running from 0 to 14 (in aqueous solutions), with 7 being neutral, numbers below 7 indicating increasing acidity, and numbers above 7 indicating increasing basicity.
A solution with a pH of 1 is not just twice as acidic as a solution with a pH of 2—it is ten times more acidic. The scale captures the enormous range of acid and base strengths in nature, from the gentle acidity of milk (pH ~6.5) to the burning power of concentrated sulfuric acid (pH ~0) and the caustic fury of drain cleaner (pH ~13-14).
Living organisms, including humans, maintain astonishingly precise pH balances within their bodies. Blood, for example, must stay around pH 7.4. Even slight deviations can lead to severe physiological consequences, highlighting how deeply the acid-base equilibrium is woven into the fabric of life.
Acids in Action
Acids are everywhere, and their influence is profound. Citric acid gives lemons their tartness. Acetic acid makes vinegar sharp and pungent. In our stomachs, hydrochloric acid helps break down food and kill pathogens.
In industry, acids are critical players. Sulfuric acid, one of the most produced chemicals worldwide, is essential for manufacturing fertilizers, refining petroleum, processing ores, and even making batteries. Nitric acid is a key ingredient in explosives and rocket fuels.
Acids can also be destructive. They corrode metals, damage living tissues, and contribute to environmental problems like acid rain, which forms when sulfur dioxide and nitrogen oxides from burning fossil fuels react with water vapor in the atmosphere, creating dilute but harmful acids that fall to the Earth.
Bases in Action
Bases, too, have their moment in the spotlight. Sodium hydroxide, known as lye, is a powerful base used in soap making, paper production, and chemical manufacturing. Ammonia, a gaseous base, finds use in fertilizers and cleaning products.
Bases are also critical in biology. Bicarbonate ions help maintain the pH of blood, preventing it from becoming too acidic. DNA and RNA, the carriers of genetic information, owe their stability to the basic properties of nitrogenous bases like adenine and guanine.
Despite their utility, bases can be just as dangerous as acids. Concentrated bases can cause severe chemical burns and must be handled with great care.
The Beauty of Neutralization
When an acid meets a base, the reaction seems simple at first glance: they neutralize each other to form water and a salt. Yet beneath this simplicity lies a profound principle of chemical balance.
Take, for instance, the reaction between hydrochloric acid and sodium hydroxide:
HCl + NaOH → NaCl + H₂O
Here, the hydrogen ion from the acid and the hydroxide ion from the base combine to form water, while the sodium and chloride ions pair off to form table salt. Energy is released in the form of heat—a small but measurable burst of the universe seeking equilibrium.
Neutralization is not just a chemical curiosity. It is essential in countless practical applications: from treating acidic soils in agriculture, to designing antacids that relieve heartburn, to purifying wastewater in environmental engineering.
Beyond Water: Acids and Bases in Other Media
While most discussions of acids and bases focus on aqueous (water-based) solutions, acid-base chemistry does not stop at water’s edge.
In non-aqueous environments—like organic solvents—acids and bases behave differently. Strong acids in water might be weak acids in acetic acid, for example, because the solvent’s properties influence how easily protons are transferred. In such environments, the definitions must be expanded beyond simple hydrogen ion transfer, leaning more heavily on the Brønsted-Lowry or Lewis concepts.
Some acids and bases even exist in the gas phase. Hydrogen chloride gas, for example, can donate protons without needing water. In these cases, chemists speak of “gas-phase acidities” and “proton affinities,” pushing the boundaries of traditional acid-base thinking.
Acids, Bases, and the Blueprint of Life
The principles of acids and bases ripple through biology with astonishing depth and subtlety.
Enzyme function, for example, often depends critically on the local pH. Many enzymes are active only within narrow pH ranges, their shapes and charge distributions delicately tuned to the acid-base environment. A shift in pH can render an enzyme useless, disrupting vital metabolic processes.
The structure of proteins—the complex molecules that do the work of life—is stabilized by intricate acid-base interactions between amino acids. Similarly, the double helix of DNA is held together by hydrogen bonds that depend on precise proton sharing between bases.
Even the communication between cells, the firing of neurons, and the contraction of muscles involve the careful orchestration of ion flows, many of which are governed by acid-base chemistry.
Without acids and bases, life as we know it could not exist.
The Power of Indicators: Seeing Acids and Bases in Color
One of the most beautiful and visually striking aspects of acid-base chemistry is the use of indicators—special substances that change color depending on the pH of their environment.
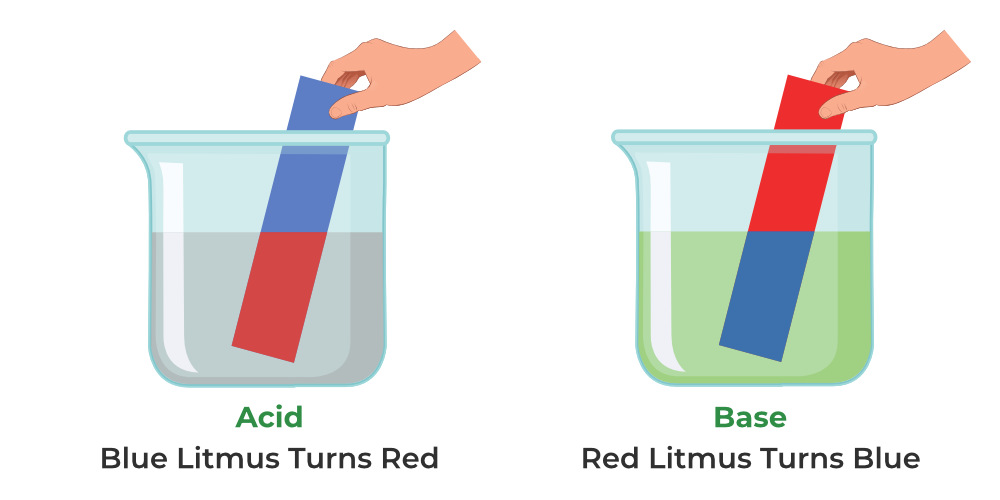
Think back to your first school science experiment, when you dipped a strip of litmus paper into a solution and watched in fascination as it turned red or blue. That simple act was your first dance with the invisible world of proton transfers.
Litmus, extracted from lichens, is a natural pH indicator: it turns red in acidic environments and blue in basic ones. But litmus is just the beginning. Phenolphthalein, for example, is colorless in acids but turns a vivid pink in bases. Bromothymol blue shifts from yellow in acidic conditions to blue in basic ones. Scientists have developed a whole rainbow of indicators, each tuned to reveal different pH ranges.
These indicators are not just educational toys. They are critical tools in medicine, environmental monitoring, and industry. A pool owner uses pH indicators to keep water safe for swimming. Doctors use blood pH indicators to diagnose respiratory or metabolic disorders. Chemists designing pharmaceuticals depend on indicators to monitor reaction conditions.
Without indicators, much of modern science would be, quite literally, colorless.
Buffers: The Guardians of Balance
Imagine a world where the slightest drop of acid could plunge an entire biological system into chaos, or a wisp of base could unravel your DNA. Life demands stability—and buffers are the unsung heroes that provide it.
A buffer is a solution that resists changes in pH when small amounts of acid or base are added. It typically consists of a weak acid and its conjugate base, or a weak base and its conjugate acid. The magic of buffers lies in their ability to “soak up” extra protons or hydroxide ions, keeping the overall pH remarkably steady.
One of the most important biological buffers is the bicarbonate system in human blood:
H₂CO₃ ⇌ H⁺ + HCO₃⁻
If the blood becomes too acidic, bicarbonate ions neutralize excess hydrogen ions. If it becomes too basic, carbonic acid releases hydrogen ions to restore balance. Thanks to this dynamic system, our blood pH stays tightly regulated around 7.4—a narrow window essential for survival.
Buffers are not just biological marvels; they are critical in industrial processes, environmental science, and even cooking. From fermentation to pharmaceuticals, the world runs smoother because buffers stand ready to defend against chaos.
Strong vs. Weak: Not Just a Matter of Concentration
When people hear the words “strong acid” or “strong base,” they often imagine something highly concentrated or dangerously corrosive. But in chemistry, “strong” and “weak” refer to how completely a substance ionizes in water, not how much of it is present.
A strong acid, like hydrochloric acid (HCl) or sulfuric acid (H₂SO₄), dissociates completely in water. Every single molecule gives up its proton, leaving no un-ionized acid behind.
A weak acid, like acetic acid (CH₃COOH), only partially dissociates. In a solution of acetic acid, most of the molecules remain intact, while only a fraction release their protons.
The same distinction holds for bases. Sodium hydroxide (NaOH) is a strong base because it completely dissociates into sodium and hydroxide ions. Ammonia (NH₃), a common weak base, only partially accepts protons to form ammonium (NH₄⁺).
Understanding this difference is crucial in science and engineering. Strong acids and bases are predictable but potentially dangerous. Weak acids and bases offer more nuanced, controllable reactions—perfect for delicate processes like biochemical reactions or drug formulation.
Amphoteric Substances: Playing Both Sides
Some substances are not content to choose sides. They can act as either an acid or a base depending on the context. These fascinating chemical chameleons are called amphoteric substances.
Water itself is the most famous example. Depending on what it encounters, water can donate a proton (acting as an acid) or accept one (acting as a base). This dual nature allows water to support countless chemical reactions vital to life.
Other examples include amino acids, the building blocks of proteins. Each amino acid has an acidic carboxyl group and a basic amino group, allowing it to engage in complex acid-base chemistry crucial to the structure and function of proteins.
Amphoterism adds a layer of flexibility and complexity to chemical systems, offering multiple pathways for reactions to proceed. It blurs the sharp line between acids and bases, revealing a spectrum rather than a simple division.
Industrial Titans: Acids and Bases in Manufacturing
The modern world would grind to a halt without acids and bases. They are the backbone of countless industrial processes, quietly working behind the scenes to create everything from fertilizer to pharmaceuticals.
Sulfuric acid alone is often called the “king of chemicals” because its production is a benchmark of industrial capacity. It is used in:
- Making fertilizers like ammonium sulfate.
- Refining petroleum into gasoline.
- Manufacturing detergents and synthetic fibers.
Bases are equally indispensable. Sodium hydroxide, or caustic soda, is a cornerstone of:
- Paper production through the Kraft process.
- Soap making via saponification of fats.
- Biodiesel manufacturing from vegetable oils.
Ammonia, although a weak base, is vital for producing nitrogen-based fertilizers that feed billions of people.
Acid-base reactions also underpin processes like electroplating, water purification, and even the extraction of metals from ores. Without these reactions, the glitter of gold and the gleam of steel would remain locked within the Earth.
Acids and Bases in Food: The Taste of Chemistry
Take a bite of a lemon, a sip of coffee, or a taste of yogurt, and you are experiencing acid-base chemistry on your tongue.
Acidity gives many foods their brightness and tang. Citric acid in citrus fruits, lactic acid in yogurt, and acetic acid in vinegar all create the sharp, refreshing flavors we crave.
Bases, while less common in food, also play key roles. Baking soda (sodium bicarbonate) is a mild base that helps baked goods rise by reacting with acids to produce carbon dioxide gas. Pretzels get their distinctive flavor and brown crust from a dip in a basic solution of lye or baking soda before baking.
Acid-base reactions are even crucial for food preservation. Pickling relies on acidic environments to inhibit microbial growth, while certain traditional cheeses use base solutions to control ripening.
Without acids and bases, the world of flavor would be a dull, uninspired place.
Environmental Impact: Acids, Bases, and the Planet
The chemistry of acids and bases extends far beyond human use—it shapes the natural world in profound ways.
Acid rain, caused by the release of sulfur dioxide and nitrogen oxides into the atmosphere, has devastated forests, lakes, and soil ecosystems. When these pollutants react with water vapor, they form sulfuric and nitric acids, falling as rain that can lower the pH of lakes to deadly levels for aquatic life.
Ocean acidification is another looming crisis. As carbon dioxide dissolves in seawater, it forms carbonic acid, lowering the ocean’s pH. This threatens coral reefs, shellfish, and the entire marine food web.
On the flip side, alkaline environments like soda lakes and alkaline soils host unique ecosystems adapted to high pH conditions. Studying these extreme environments offers insights into the resilience of life and clues to the possibilities of life on other planets.
Understanding and managing acid-base balance is critical not only for individual health but for the health of our planet.
The Future of Acid-Base Chemistry
As science advances, so does our understanding of acids and bases. New frontiers are opening up, including:
- Superacids: Acids stronger than pure sulfuric acid, capable of protonating even seemingly inert substances.
- Superbases: Bases so strong they can rip protons from almost anything, used in cutting-edge organic synthesis.
- Ionic liquids: Salts that are liquid at room temperature, offering new possibilities for acid-base reactions without water.
Researchers are also exploring green chemistry approaches to acid-base reactions, aiming to design processes that minimize waste, energy use, and environmental impact.
Acid-base chemistry is evolving, but its fundamental power—its ability to rearrange matter with the simple movement of protons and electrons—remains eternal.
Conclusion: The Eternal Dance of Acids and Bases
From the ancient alchemists to the modern laboratories pushing the boundaries of knowledge, the story of acids and bases is one of endless fascination and discovery. These substances, so different yet so intimately connected, define the chemistry of life, industry, environment, and the cosmos itself.
Acids and bases are not just “chemicals.” They are a way of understanding how change happens—how structures are built and broken, how balance is achieved and lost, how simplicity gives rise to complexity.
Every sour taste, every slippery touch, every heartbeat, and every breath carries echoes of the great dance between acids and bases. Invisible yet ever-present, they remind us that the universe’s greatest secrets are often hidden in the smallest exchanges.
And so, the next time you squeeze a lemon, scrub a floor, or breathe deeply after a rainstorm, remember: you are part of the endless, beautiful, and profoundly important dance of acids and bases.
Behind every word on this website is a team pouring heart and soul into bringing you real, unbiased science—without the backing of big corporations, without financial support.
When you share, you’re doing more than spreading knowledge.
You’re standing for truth in a world full of noise. You’re empowering discovery. You’re lifting up independent voices that refuse to be silenced.
If this story touched you, don’t keep it to yourself.
Share it. Because the truth matters. Because progress matters. Because together, we can make a difference.
Your share is more than just a click—it’s a way to help us keep going.