Ammonia (NH3) is an essential chemical widely used in fertilizers, industrial processes, and as a precursor for many chemicals. The conventional method for producing ammonia, the Haber-Bosch process, has been the cornerstone of global ammonia synthesis for over a century. This energy-intensive process involves converting nitrogen (N2) and hydrogen (H2) into ammonia at high temperatures (400–500℃) and pressures (10–30 MPa). Despite being crucial for feeding a growing global population, the Haber-Bosch process has substantial downsides: it accounts for about 1%–2% of global energy consumption and is responsible for nearly 1% of the world’s carbon dioxide (CO2) emissions, making it unsustainable in the context of modern environmental concerns.
In recent years, alternative approaches to ammonia synthesis have gained attention, particularly methods that aim to reduce the environmental footprint of production. One such promising route is the electrocatalytic nitrate reduction reaction (NO3−RR), which harnesses renewable energy to drive ammonia production. This method uses nitrate (NO3−) from wastewater as the nitrogen source and water (H2O) as the hydrogen source, potentially offering a low-carbon, sustainable alternative under much milder operating conditions.
Electrocatalytic nitrate reduction holds great promise but has been plagued by significant challenges. Two critical issues have limited its broader application: the unsatisfactory electrocatalytic activity of the materials used and the inability to maintain stable performance over long durations. Both factors hinder the development of this process into a commercially viable alternative to the traditional Haber-Bosch method.
In an exciting recent development, researchers at the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), led by Professors Gao Dunfeng, Wang Guoxiong, and Bao Xinhe, have made significant strides in overcoming these limitations. Their breakthrough, detailed in a study published in Nature Communications, introduces a novel approach for ammonia electrosynthesis by employing a dual-phase Cu foam electrode. The electrode significantly enhances the efficiency of nitrate reduction to ammonia, offering both high catalytic activity and remarkable long-term stability.
New Electrode Design
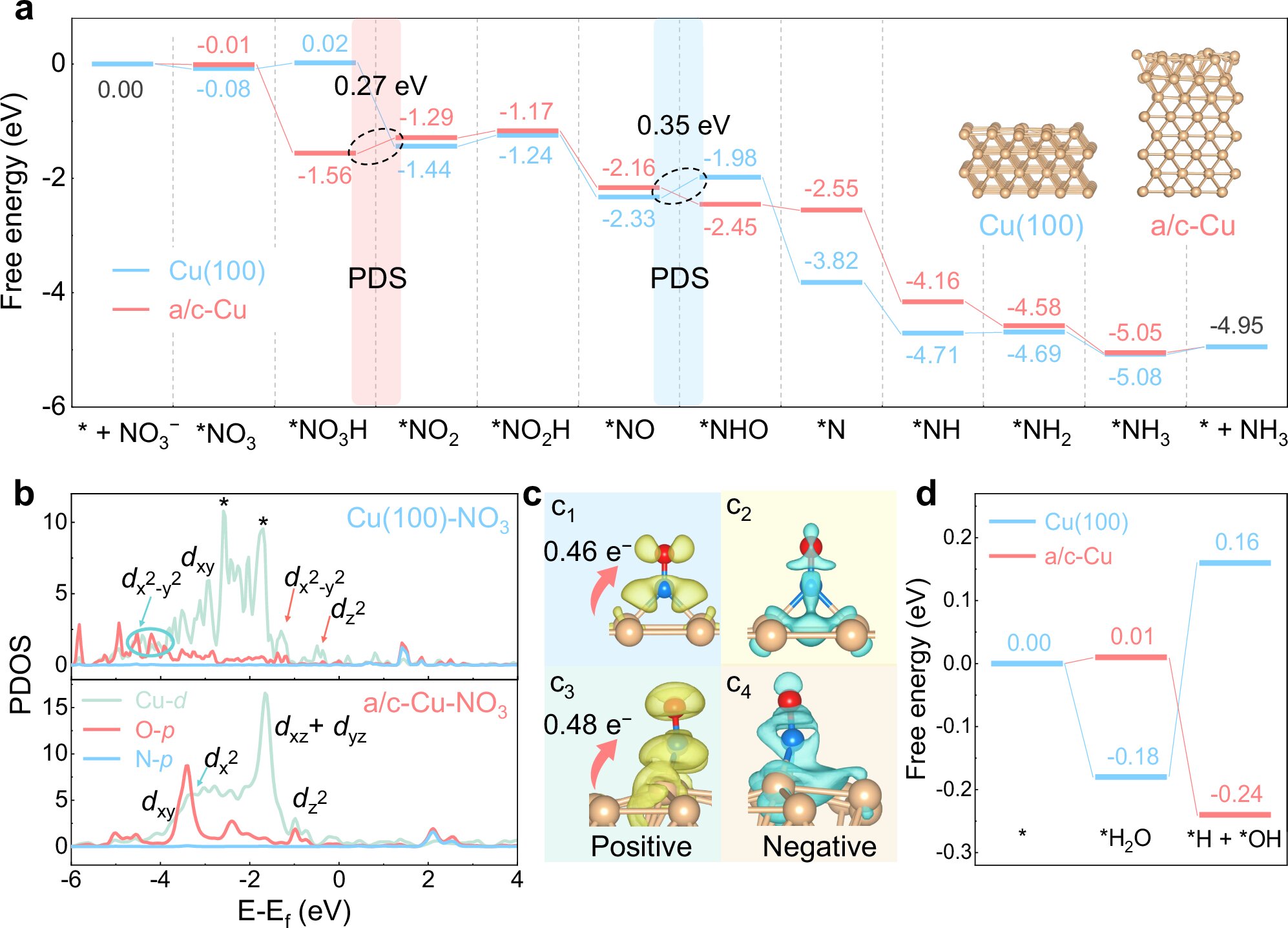
The research team developed a unique amorphous/crystalline dual-phase copper (Cu) foam electrode. This was achieved by subjecting commercial Cu foam to thermal annealing in air, which created a dual-phase structure consisting of both crystalline and amorphous Cu domains. This innovative design not only improved the performance of the electrocatalyst but also made it highly durable. The use of copper foam, a readily available and cost-effective material, contributes to the scalability and feasibility of the approach.
High Performance and Stability
When tested in an alkaline membrane electrode assembly (MEA) electrolyzer, the researchers achieved significant results. The electrode demonstrated an impressive NH3 partial current density of 3.33 A/cm2 and an NH3 formation rate of 15.5 mmol/h/cm2 at a cell voltage of just 2.6 V. Remarkably, the electrode maintained stable ammonia production at a Faradaic efficiency of around 90% when an applied current density of 1.5 A/cm2 was maintained over a prolonged period of 300 hours. This stability is a crucial aspect of its potential for long-term, commercial-scale application.
The high Faradaic efficiency signifies that most of the applied electrical energy is being utilized to produce ammonia, minimizing energy losses in the process. Additionally, the durability observed in the experiments marks a significant step toward overcoming the issue of long-term stability that has historically hindered the commercial implementation of electrocatalytic nitrate reduction.
Insights into Catalytic Mechanisms
Through their research, the team also made important discoveries about the catalytic mechanisms at play. They found that the stable amorphous Cu domains on the electrode surface played a crucial role in the exceptional electrocatalytic activity. The team proposed that the unique structure of the dual-phase Cu material facilitates the optimal adsorption and reduction of nitrate, leading to higher efficiency and greater stability. These insights could help further optimize electrocatalytic materials for nitrate reduction in the future.
In addition to the impressive performance in lab-scale electrolysis, the researchers conducted a scale-up demonstration using a larger electrode (100 cm²), producing ammonia at a formation rate of up to 11.9 g/h with an applied current of 160 A. This further emphasizes the potential for scaling this method to larger, industrially relevant setups while maintaining high rates of ammonia production.
Significance for Sustainable NH3 Production
This work presents a promising low-carbon alternative for ammonia production. By utilizing nitrate from wastewater as the nitrogen source and water as the hydrogen source, the electrocatalytic nitrate reduction method offers a way to synthesize ammonia at much lower environmental and energy costs compared to traditional methods. The use of a relatively simple and inexpensive material—copper foam—as the electrode catalyst is also a key factor that makes the process more economically feasible.
“This work also underscores the importance of stabilizing metastable amorphous structures for improving electrocatalytic reactivity and long-term stability,” said Prof. Wang Guoxiong, one of the lead authors of the study. The ability to stabilize metastable phases in catalysts, as demonstrated in this research, could be a key to advancing not just ammonia electrosynthesis, but many other energy-intensive chemical processes that need to transition toward more sustainable practices.
Future Implications
The discovery of this high-performance, dual-phase Cu foam electrode marks a significant advance in the field of ammonia electrosynthesis and presents a new path forward for environmentally sustainable ammonia production. Future work will likely focus on further optimizing the catalysts to improve performance at even larger scales, fine-tuning the electrode’s design for maximum efficiency, and integrating the process with renewable energy sources like wind, solar, or hydroelectric power to create a truly sustainable system for ammonia synthesis.
The progress made by this research team serves as a shining example of how scientific ingenuity can address some of the world’s most pressing challenges, such as climate change and the need for cleaner industrial processes. By moving beyond traditional methods and exploring alternative, renewable-driven processes, researchers are helping pave the way for more sustainable practices across many sectors, particularly in agriculture, where ammonia plays such a crucial role as a fertilizer.
The electrocatalytic nitrate reduction process, if scaled to industrial levels, could dramatically reduce the carbon footprint of ammonia production, contributing to a cleaner and more sustainable global economy. With the work of the DICP team, the dream of a cleaner, greener method for synthesizing ammonia may not be far from becoming a practical reality.
Reference: Yi Wang et al, Ammonia electrosynthesis from nitrate using a stable amorphous/crystalline dual-phase Cu catalyst, Nature Communications (2025). DOI: 10.1038/s41467-025-55889-9