The mammalian brain is a marvel of evolution, a living network teeming with dynamic processes that allow humans and animals alike to navigate an endlessly changing world. One of its most extraordinary feats is the construction of cognitive maps—internal representations of spatial environments that help guide movements, memories, and decision-making. Nestled within the brain’s hippocampus, a structure long associated with memory and navigation, are specialized neurons known as place cells (PCs). These cells, particularly abundant in the CA1 region of the hippocampus, have captivated neuroscientists since their discovery, thanks to their unique ability: they activate when an animal occupies or visits a particular location in its environment.
Over the decades, the scientific community has pieced together the idea that the activation of place cells encodes information about both space and goals, forming the scaffold of cognitive maps that allow organisms to move purposefully through the world. Yet one pivotal question remained elusive: How exactly does experience shape these cognitive maps? Are they rigid blueprints laid down once and for all, or are they flexible frameworks continually molded by an animal’s encounters and goals?
In a groundbreaking study published recently in Nature Neuroscience, researchers from Baylor College of Medicine—Fish Kunxun Qian, Yiding Li, and Jeffrey C. Magee—ventured into the depths of this mystery. Their findings reveal a breathtakingly adaptive brain mechanism: experiences can fine-tune the synaptic inputs that place cells receive, dynamically reshaping the brain’s internal maps to better meet the demands of a shifting environment.
A Window into the Brain’s Navigational Machinery
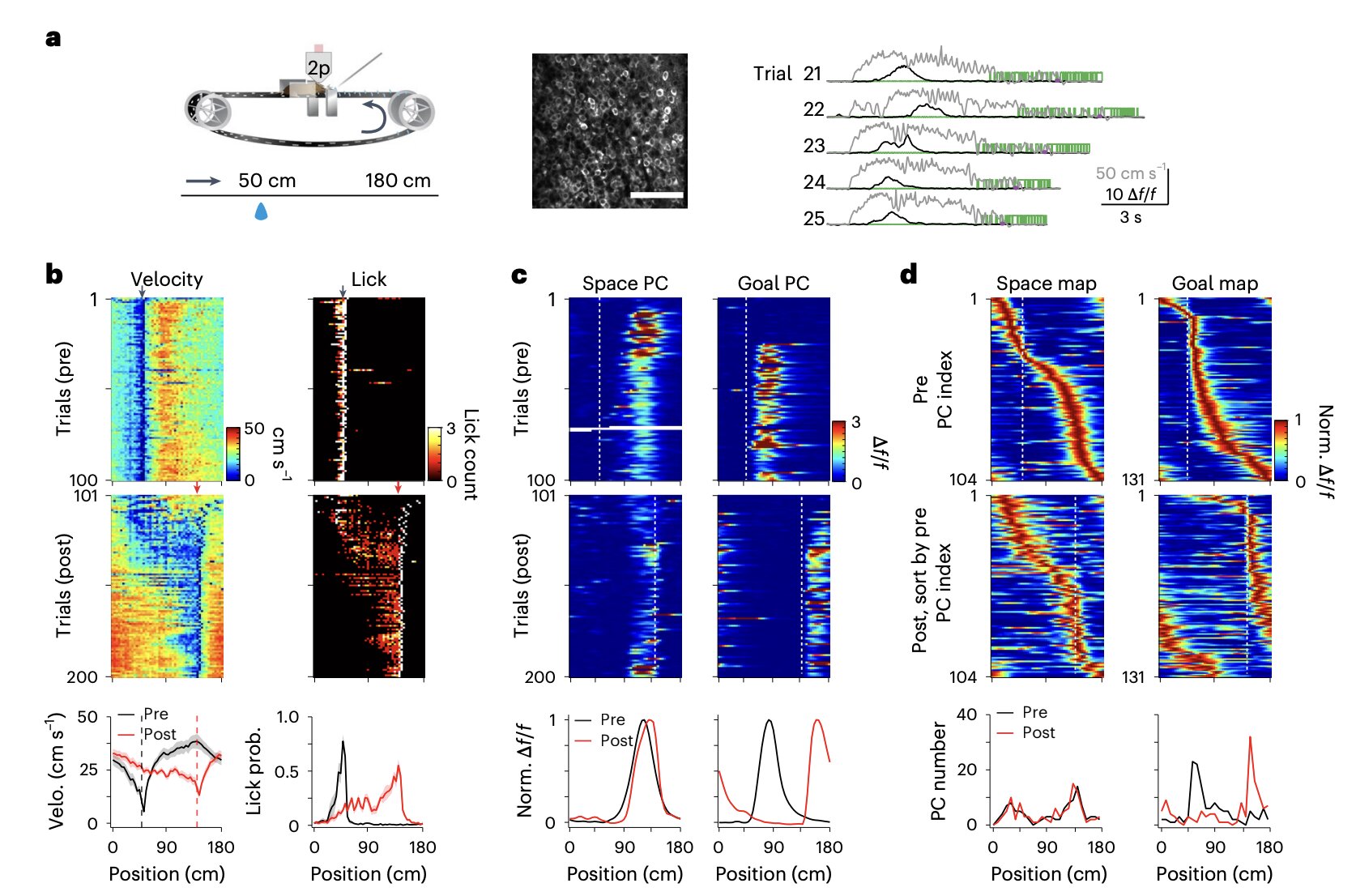
To investigate the relationship between experience and cognitive mapping, Qian, Li, and Magee designed a study that pushed the limits of current technology and methodology. They employed longitudinal recordings of place cell activity in live, head-fixed mice engaged in a spatial learning task performed on a treadmill. This approach offered an exquisite level of control over the experimental environment while allowing the researchers to capture the subtle nuances of how the brain encodes space and goals.
Two powerful techniques were utilized to peer into the hippocampal CA1 region: two-photon imaging, which enables the visualization of active neurons with remarkable clarity, and in vivo intracellular recordings via implanted electrodes, offering a direct measure of the electrical dance within individual cells. This dual-pronged strategy allowed the team not only to observe when and where the place cells fired but also to dissect the underlying synaptic inputs guiding their behavior.
Their study involved 22 adult mice as they navigated both familiar and novel environments. By carefully manipulating environmental familiarity and observing corresponding neuronal changes, the researchers could pinpoint how experience modified the internal maps represented by place cell networks.
Flexible Frames of Reference: Space and Goals in the Hippocampus
As the mice traversed a familiar environment, their CA1 place cells painted a balanced cognitive landscape. Roughly half of the active place cells were space-referenced, firing at specific physical locations, while the other half were goal-referenced, firing based on proximity to a reward or objective. This dual coding suggests that even under stable conditions, the brain maintains a flexible balance between where the animal is and where it needs to go.
However, when the animals were introduced to a novel environment, the landscape inside their brains shifted dramatically. Space-referenced cells, once devoted to physical locations, adaptively switched their reference frames to become goal-referenced. In other words, when the mice found themselves in an unfamiliar world, their hippocampal networks prioritized reaching goals over adhering to a strict spatial grid. The cognitive map itself bent to the demands of the moment, becoming a tool for successful navigation rather than a rigid representation of reality.
This remarkable adaptability underscores a critical insight: experience doesn’t merely populate the brain’s maps with new locations—it actively reconfigures the underlying structure of the maps themselves to optimize behavior.
Peering Deeper: The Synaptic Symphony Behind Flexible Mapping
The Baylor team didn’t stop at identifying these shifts in place cell behavior; they dug even deeper to uncover the synaptic underpinnings of this phenomenon. By recording the intracellular membrane potentials of individual CA1 neurons, they revealed a fascinating orchestration of synaptic inputs.
Every CA1 place cell simultaneously received both space-referenced and goal-referenced synaptic inputs. The secret to whether a particular cell favored space or goals lay in the ratio of these competing inputs. Cells with stronger goal-referenced inputs were more likely to shift toward goal-referencing, particularly in novel environments.
Moreover, these ratios were not static. Instead, they were shaped through a phenomenon known as behavioral timescale synaptic plasticity—a rapid form of synaptic change that occurs during behaviorally relevant experiences. As mice learned and adapted to new conditions, their place cells’ synaptic inputs dynamically adjusted, tweaking the balance between space and goal signals to optimize navigation.
Thus, experience actively sculpts the cognitive map by fine-tuning the synaptic inputs that drive the activity of place cells. Rather than being fixed entities, cognitive maps are living, breathing constructions that flex and adapt with every new experience.
A New Paradigm for Understanding Spatial Learning and Memory
The implications of these findings stretch far beyond mice running on treadmills. They illuminate a fundamental principle of how the brain organizes knowledge: flexibility is the hallmark of intelligence. The brain’s ability to adjust its internal representations in response to changing goals and experiences could explain not only spatial learning but also broader aspects of memory, decision-making, and even problem-solving.
Moreover, the Baylor study offers tantalizing clues for unraveling the mysteries of neurological conditions where cognitive mapping goes awry. Disorders such as Alzheimer’s disease, schizophrenia, and post-traumatic stress disorder often involve impairments in spatial memory and goal-directed behavior. Understanding how synaptic inputs dynamically shape cognitive maps may one day lead to new therapeutic strategies for restoring or enhancing cognitive flexibility in such conditions.
The Future of Cognitive Map Research
While Qian, Li, and Magee’s study represents a major leap forward, it also opens a host of new questions. What are the molecular signals that trigger the adjustment of synaptic ratios? How do different types of experiences—positive, negative, stressful—affect the flexibility of cognitive maps? Could manipulating synaptic input ratios artificially enhance learning or memory performance?
Future studies will likely explore these avenues, leveraging ever more sophisticated imaging and recording technologies. Researchers may also begin to test whether similar mechanisms operate in other brain regions or in more complex behaviors beyond spatial navigation.
Ultimately, these investigations promise not only to deepen our understanding of the brain’s remarkable plasticity but also to bring us closer to unlocking the full potential of the mind.
Conclusion: A Brain Built for Change
The Baylor College of Medicine study shatters the notion of the brain as a static mapmaker. Instead, it reveals a brain that is built for change, whose maps are as dynamic as the landscapes they represent. Through the adaptive adjustment of synaptic inputs to place cells, experience molds and reshapes the cognitive maps that allow creatures great and small to navigate a world that is ever in flux.
In the intricate ballet of neurons and synapses, every step taken, every new path explored, leaves an indelible mark on the mind’s internal cartography. Our memories, our goals, our very sense of place in the world—all are written not in stone, but in the living, breathing language of experience.
Reference: Fish Kunxun Qian et al, Mechanisms of experience-dependent place-cell referencing in hippocampal area CA1, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-01930-5.
Behind every word on this website is a team pouring heart and soul into bringing you real, unbiased science—without the backing of big corporations, without financial support.
When you share, you’re doing more than spreading knowledge.
You’re standing for truth in a world full of noise. You’re empowering discovery. You’re lifting up independent voices that refuse to be silenced.
If this story touched you, don’t keep it to yourself.
Share it. Because the truth matters. Because progress matters. Because together, we can make a difference.
Your share is more than just a click—it’s a way to help us keep going.