Superionic materials are a fascinating class of compounds that blur the lines between solids and liquids. They exhibit a unique combination of solid-like atomic structure and liquid-like ionic mobility. This dual nature makes superionic materials particularly intriguing for various advanced technologies, especially in the field of energy storage. Solid-state batteries, which use solid electrolytes instead of liquid ones, could greatly benefit from these materials due to their exceptional ionic conductivity. However, despite their potential, the fundamental physics governing the rapid ionic diffusion in superionic materials is not fully understood. Recent research conducted by scientists at Duke University and other research institutes has shed light on the mechanisms that govern ion mobility in a specific superionic compound, Li₆PS₅Cl, and its implications for improving solid-state batteries.
What Are Superionic Materials?
Superionic materials are substances that exhibit a combination of solid-like structure and liquid-like behavior, specifically when it comes to the movement of ions. In these materials, ions—charged atoms or molecules—can move more freely than they would in a typical solid, resembling the behavior seen in liquids. However, the material’s overall atomic structure remains solid, meaning it maintains a crystalline arrangement. This behavior allows ions to diffuse rapidly through the material, which is an essential characteristic for applications like solid-state batteries.
In a solid-state battery, instead of relying on a liquid electrolyte to facilitate the movement of ions between the anode and cathode, a solid electrolyte is used. Solid-state batteries promise higher energy densities, better safety, and longer lifetimes compared to conventional lithium-ion batteries that use liquid electrolytes. However, the performance of solid-state batteries is highly dependent on the ionic conductivity of the electrolyte. This is where superionic materials come in, as they possess the high ionic conductivity necessary for efficient energy storage and conversion.
The Mystery of Ionic Diffusion in Superionic Materials
Despite extensive research, the precise mechanisms that enable the rapid ionic diffusion in superionic materials remain poorly understood. Researchers have debated whether this high ionic conductivity arises from the liquid-like dynamics of the ions or from the phonons—the quantized vibrations of atoms in a crystalline lattice—typically found in solids.
To address this uncertainty, researchers have focused on the compound Li₆PS₅Cl, a lithium-based superionic material that has shown promise as a potential solid-state electrolyte. Previous studies had suggested that Li₆PS₅Cl exhibits exceptional ionic conductivity, but the underlying physics were not fully clear. Some scientists believed that the movement of ions in Li₆PS₅Cl could be explained by traditional lattice dynamics (phonons), while others suspected that the ionic behavior might be more akin to the free movement of ions in a liquid.
A recent study led by Olivier Delaire, a professor at Duke University, aimed to resolve this ambiguity by investigating the dynamics of Li+ ions in Li₆PS₅Cl. Their findings, published in Nature Physics, provide new insights into the nature of ionic diffusion in superionic materials, offering valuable clues for optimizing future solid-state batteries.
Investigating the Dynamics of Li₆PS₅Cl
To investigate the ion mobility in Li₆PS₅Cl, Delaire and his team used a combination of neutron scattering experiments and atomistic computer simulations. Neutron scattering is a powerful technique used to study atomic vibrations and dynamics. By using neutron scattering, the researchers could observe the behavior of Li+ ions at the atomic level, including their movements within the material’s crystalline framework. Neutron scattering is particularly effective for probing atomic motions with frequencies in the GHz-THz range, which corresponds to the kinds of vibrations and motions that occur at the atomic level.
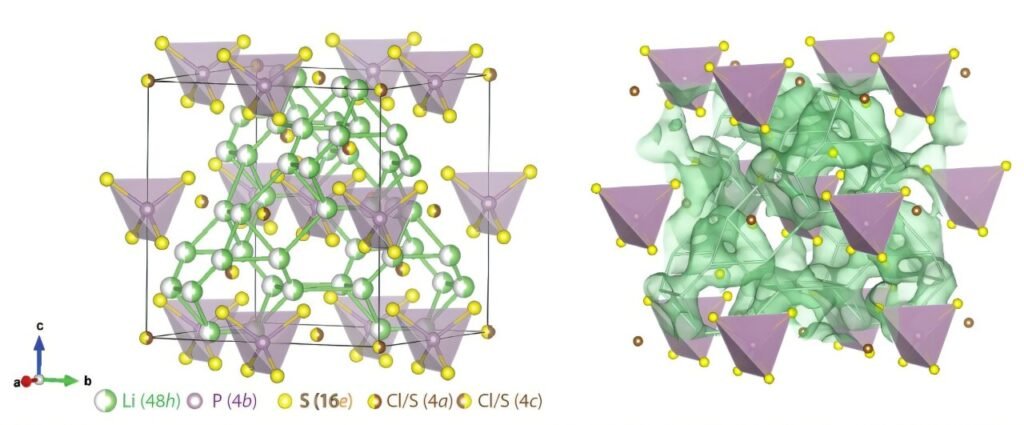
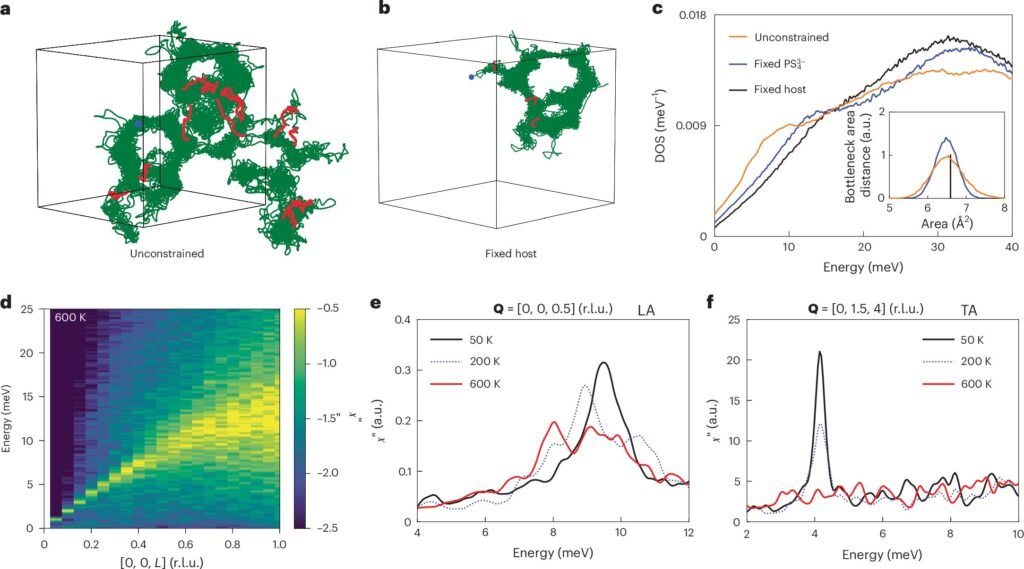
In addition to neutron scattering, the team employed first-principles computer simulations, which are based on quantum mechanical principles and augmented with machine learning techniques. These simulations helped the researchers model and predict the ion dynamics in the material, allowing them to compare experimental data with theoretical predictions.
One of the key questions the researchers sought to answer was whether the rapid ion diffusion in Li₆PS₅Cl could be explained by traditional lattice vibrations (phonons) or if it was due to a more liquid-like motion of the ions. The researchers observed that as the Li+ ions moved through the crystalline structure, their vibrational spectra shifted from a crystal-like behavior to a more liquid-like pattern. This suggests that the ions were not simply vibrating in place but were hopping between different sites in the structure, similar to the way ions would move in a liquid.
The combination of neutron scattering and machine learning-based molecular dynamics simulations allowed the team to observe how the vibrational modes of the crystal framework and the movements of the diffusing ions were closely linked. This insight suggests that the rapid ionic motion in Li₆PS₅Cl is not just a result of phonon activity in the crystal but also involves liquid-like ion mobility.
Implications for Solid-State Batteries
The findings from this study are highly significant for the development of solid-state batteries. For a solid-state electrolyte to perform well in a battery, it must exhibit high ionic conductivity—the ability to move ions efficiently between the anode and cathode—while maintaining the stability of the solid structure. Li₆PS₅Cl has shown promising ionic conductivity, but its true potential can only be realized if its mechanisms of ion mobility are fully understood and optimized.
The new insights into how Li+ ions diffuse in Li₆PS₅Cl open up new avenues for designing materials with high ionic conductivity and good stability. The research highlights the importance of considering both the ion dynamics and the vibrational modes of the host crystal in designing future materials for energy storage applications.
Delaire and his team believe that understanding the combined dynamics of diffusive ions and the vibrational modes of the crystal framework could lead to the design of more efficient superionic materials. The goal is to create materials that exhibit both high ionic diffusivity (comparable to liquids) and excellent stability, making them ideal for solid-state batteries and other energy storage devices.
The Role of Machine Learning in Materials Research
One of the most exciting aspects of this research is the use of machine learning and artificial intelligence in materials science. By leveraging these technologies, Delaire’s team was able to accelerate their simulations and improve the accuracy of their predictions. Machine learning techniques allow researchers to train models on large datasets, such as molecular dynamics simulations, and use these models to predict the behavior of new materials. This approach is particularly valuable in materials science, where the vast number of possible material compositions makes it difficult to screen for optimal candidates manually.
Furthermore, advancements in neutron scattering instruments, combined with machine learning, could drastically accelerate the discovery and optimization of superionic materials. In the future, researchers hope to use these tools to screen many different compositions quickly, identifying the best candidates for applications in solid-state batteries, fuel cells, and even neuromorphic computing hardware.
Conclusion: The Future of Superionic Materials
The recent study on Li₆PS₅Cl marks an important step forward in understanding the behavior of superionic materials. By revealing that the rapid ionic diffusion in this compound is driven by liquid-like dynamics, the research opens up new possibilities for optimizing solid-state batteries and other energy storage devices.
As we move toward a future of renewable energy and electric vehicles, the need for more efficient, long-lasting energy storage solutions will only grow. Superionic materials, with their unique combination of solid and liquid characteristics, could be the key to unlocking this future. The continued development of these materials, coupled with advances in computational techniques and machine learning, holds the potential to revolutionize energy storage and power conversion technologies in the years to come.
As Delaire and his team continue to explore new superionic material compositions, they are optimistic about the possibilities that lie ahead. Their work is paving the way for new materials that could transform not only batteries but also a wide range of other technologies that rely on efficient energy storage and conversion. The next decade could see a rapid acceleration in the development of superionic materials, ultimately leading to more efficient, sustainable, and advanced technologies.
Reference: Jingxuan Ding et al, Liquid-like dynamics in a solid-state lithium electrolyte, Nature Physics (2025). DOI: 10.1038/s41567-024-02707-6
