When we think of genetics, we often picture the sequence of DNA that makes us who we are. The genetic code—a blueprint for life—is written in the nucleotides of our DNA, guiding the development, function, and reproduction of every cell in our body. It’s the fundamental code, passed down from our parents, that determines eye color, hair texture, and susceptibility to certain diseases.
But what if we told you that our genes don’t always tell the whole story? What if there’s another layer of information that influences how our genes express themselves—information that can be passed down to future generations, even though it doesn’t change the genetic code itself? This is where epigenetics comes into play.
Epigenetics is the study of changes in gene expression that do not involve alterations to the underlying DNA sequence. These changes are influenced by a variety of environmental factors and life experiences, such as diet, stress, exposure to toxins, and even childhood trauma. Epigenetics challenges the traditional idea that our genetic fate is set in stone. Instead, it suggests that we have a dynamic relationship with our genetic material—a relationship that can be influenced and shaped by external forces.
In this article, we will explore the fascinating world of epigenetics, uncovering how it works, how it influences health and disease, and why it could hold the key to understanding some of the most complex biological processes.
The Epigenome: A Layer of Control
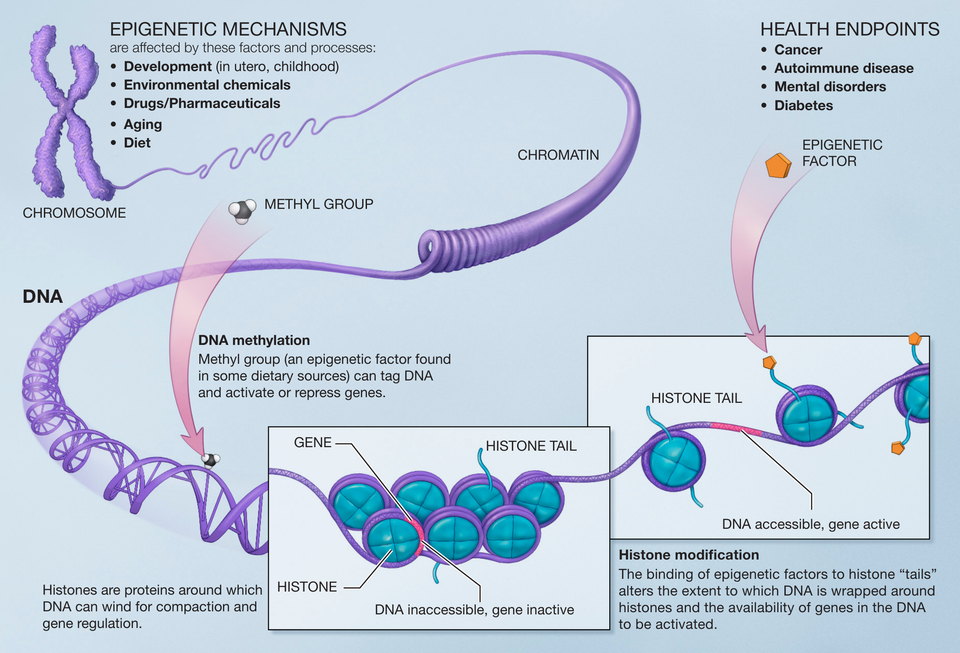
To understand epigenetics, we need to first grasp the concept of the epigenome—a collection of chemical modifications to our DNA and the proteins it interacts with. The term “epigenetics” itself is derived from the Greek words “epi,” meaning “over” or “above,” and “genetics,” referring to the genes themselves. In essence, epigenetics is the study of changes that occur on top of the genetic code, influencing how genes are turned on or off.
The epigenome consists of a variety of chemical tags and modifications that regulate gene expression. These modifications do not change the DNA sequence itself but instead influence how accessible the genes are to the cellular machinery responsible for reading and interpreting them. The two most common types of epigenetic modifications are DNA methylation and histone modification.
DNA Methylation: Adding a Chemical Tag
One of the most studied forms of epigenetic regulation is DNA methylation, where a methyl group (a small chemical compound consisting of one carbon and three hydrogen atoms) is added to certain sites on the DNA molecule. Specifically, methylation often occurs at cytosine bases, one of the four nucleotide bases that make up the DNA code.
DNA methylation typically leads to gene silencing, essentially turning off the expression of a gene. When methyl groups are added to the promoter region of a gene (the part of the gene that controls its expression), it prevents the gene from being activated, meaning the cell does not produce the protein that the gene codes for. This is a key mechanism for regulating which genes are turned on and off in different cells, tissues, and organs.
Methylation patterns can be influenced by a variety of environmental factors, such as diet, stress, and exposure to toxins. For example, studies have shown that a high-fat diet can lead to changes in the DNA methylation patterns of certain genes involved in metabolism, which may contribute to the development of obesity or diabetes.
Histone Modification: Tying Up Genes in a Nucleosome
Another important aspect of the epigenome involves the modification of histones—the proteins around which DNA is wrapped in the cell nucleus. DNA is packaged into a compact structure called chromatin, and histones play a crucial role in this process. Just as spools help organize thread, histones help organize DNA within the cell. However, the DNA wrapped around these histones must be able to be “unwrapped” in order for the cell to read and express the genes.
Histones can be chemically modified in a variety of ways, such as through the addition of acetyl groups, methyl groups, or phosphate groups. These modifications can either loosen or tighten the chromatin structure, making the DNA more or less accessible to the cellular machinery that reads and transcribes genes. For example, when histones are acetylated, the chromatin becomes less tightly packed, allowing genes to be more easily expressed. On the other hand, when histones are methylated, the chromatin becomes more compact, which can silence gene expression.
Histone modifications also play a role in processes like DNA repair, replication, and cell division. Like DNA methylation, histone modifications can be influenced by environmental factors and may be passed down from one generation to the next.
The Dynamic Nature of the Epigenome
Unlike the genome, which is relatively stable, the epigenome is highly dynamic. Epigenetic modifications can change throughout an individual’s lifetime in response to environmental factors and experiences. This flexibility allows the body to adapt to changing conditions, but it also means that the epigenome can be vulnerable to long-term environmental influences.
For example, studies have shown that early-life stress can lead to long-lasting changes in DNA methylation patterns, affecting the expression of genes involved in stress response and mental health. Similarly, exposure to environmental toxins like tobacco smoke or air pollution can alter the epigenome in ways that increase the risk of diseases such as cancer and cardiovascular disease.
The fact that the epigenome is reversible is both a blessing and a curse. On one hand, it means that the body can respond to changes in the environment by adjusting gene expression. On the other hand, it also means that environmental exposures—such as poor diet, lack of exercise, or exposure to harmful chemicals—can leave lasting marks on our epigenome, potentially influencing our health for years to come.
Epigenetics and Disease: The Role of Environmental Factors
One of the most exciting aspects of epigenetics is its potential to explain how environmental factors contribute to the development of diseases. While genetics certainly plays a major role in conditions like cancer, heart disease, and diabetes, epigenetics adds another layer of complexity by showing how external influences can affect gene expression in ways that increase disease risk.
Cancer: The Epigenetic Origins of Tumors
Cancer is one of the most well-studied diseases in the context of epigenetics. Traditionally, cancer has been understood as a genetic disease, caused by mutations in specific genes that drive uncontrolled cell growth. However, epigenetic changes can also contribute to the development of cancer by turning on or off genes that regulate cell division, apoptosis (programmed cell death), and DNA repair.
In many cases, cancer cells exhibit widespread changes in DNA methylation and histone modification patterns. For example, tumor suppressor genes—genes that normally help prevent uncontrolled cell growth—may be silenced through DNA methylation, allowing cancerous cells to proliferate unchecked. Similarly, genes that promote cell division may be turned on by epigenetic modifications, further driving tumor growth.
These epigenetic changes are often reversible, which means that they could potentially be targeted with new therapies. In fact, several drugs that target the epigenome are currently being tested in clinical trials, offering hope for new treatments that could specifically reverse the epigenetic changes associated with cancer.
Neurological Diseases: The Epigenetic Basis of Brain Disorders
Epigenetics also plays a significant role in neurological diseases, particularly those related to mental health and neurodevelopmental disorders. Conditions such as autism, schizophrenia, and depression have long been thought to have a genetic basis, but researchers are now uncovering how environmental factors can alter the epigenome in ways that influence brain development and function.
For instance, prenatal exposure to stress, toxins, or poor nutrition can lead to epigenetic changes in the developing brain, potentially increasing the risk of mental health disorders later in life. In animal studies, researchers have shown that changes in DNA methylation and histone modification patterns in the brain can affect behavior, cognition, and emotional regulation.
In some cases, epigenetic changes have been linked to the expression of specific genes involved in neurotransmitter signaling, synaptic plasticity, and neural development. These findings have opened up new avenues for potential treatments for conditions like depression and anxiety, which are often difficult to treat with traditional therapies.
Cardiovascular Disease: How the Epigenome Affects Heart Health
Cardiovascular disease (CVD) is another area where epigenetics plays a crucial role. Like cancer, heart disease has long been understood as a genetic and environmental disease, with risk factors such as diet, exercise, and smoking influencing an individual’s likelihood of developing the condition. However, epigenetic modifications also play a part in regulating genes involved in blood pressure, cholesterol metabolism, and inflammation.
For example, studies have shown that DNA methylation can affect genes that regulate vascular function, leading to changes in blood vessel constriction and dilation. Similarly, histone modifications can influence the expression of genes involved in inflammation, a key factor in the development of atherosclerosis (plaque buildup in the arteries). These findings suggest that epigenetic therapies could one day be used to prevent or treat cardiovascular disease by targeting the epigenetic changes that contribute to the condition.
Epigenetics and Inheritance: The Legacy of Our Environment
Perhaps one of the most profound implications of epigenetics is the potential for intergenerational inheritance—the idea that epigenetic changes can be passed down from one generation to the next, even though they do not involve changes to the underlying DNA sequence. This concept challenges the traditional view of inheritance, which holds that only genetic information is passed from parent to child.
Studies in animals have shown that environmental factors such as diet, stress, and toxins can lead to epigenetic changes in the germline (the cells that give rise to eggs and sperm), which can then be passed on to offspring. This means that experiences and exposures from one generation could potentially influence the health of future generations, even without altering the DNA code.
For example, research has shown that mice exposed to a high-fat diet can pass on epigenetic changes to their offspring that affect metabolism and obesity risk. Similarly, studies in humans have suggested that childhood trauma or malnutrition can lead to epigenetic changes that affect mental health and disease susceptibility in subsequent generations.
The implications of intergenerational epigenetic inheritance are profound, raising questions about how environmental exposures and lifestyle choices can impact not only our health but the health of our descendants.
Conclusion: The Future of Epigenetics – Unlocking the Secrets of Life
Epigenetics has opened up a new frontier in our understanding of biology, revealing the dynamic, adaptable nature of our genome. It shows us that our genetic fate is not set in stone and that environmental factors, life experiences, and even the choices we make can influence gene expression in profound ways. The power of epigenetics lies in its ability to explain the complex interactions between our genes and our environment, shedding light on the molecular basis of disease, development, and behavior.
As research continues to uncover the mechanisms behind epigenetic regulation, it holds the promise of new therapies for a wide range of diseases, from cancer to mental health disorders to cardiovascular disease. It may also offer new insights into aging, longevity, and how we can optimize our health at every stage of life.
The future of epigenetics is one of profound discovery, offering the possibility of unlocking the secrets of life itself. As we continue to explore this fascinating field, we may just be on the cusp of uncovering a new chapter in the story of human health, inheritance, and evolution.