In an exciting advancement in cancer immunotherapy, a research team at the Medical University of Vienna, led by Maria Sibilia, has explored a novel combination therapy that may significantly improve outcomes for patients with certain types of cancer, such as melanoma and breast cancer. The therapy combines the systemic administration of interferon-I, a tissue hormone, with a local application of Imiquimod, a known immune-modulating agent. Recent findings published in Nature Cancer suggest that this combination therapy has the potential to effectively target superficial tumors while also fighting distant metastases, offering hope to patients whose cancers have not been sufficiently addressed by current treatments.
The Need for New Cancer Treatments
In recent years, immunotherapy has revolutionized cancer treatment. This approach works by harnessing the power of the immune system to identify and destroy cancer cells, often yielding impressive results. Immunotherapies, including checkpoint inhibitors, have helped many patients experience long-lasting remission. However, immunotherapies are not universally effective, and certain cancer types or patient groups still struggle with resistance to these treatments.
Maria Sibilia, Head of the Center for Cancer Research at the Medical University of Vienna, is spearheading efforts to tackle this issue. Her research investigates how a combination of traditional and innovative immunotherapeutic agents might be used to enhance effectiveness, especially in tumors that have proven difficult to treat. In her recent preclinical study, Sibilia and her team explored a new approach using interferon-I (IFN-I) and Imiquimod, focusing on melanoma and breast cancer as model diseases.
The Basics of the New Combination Therapy
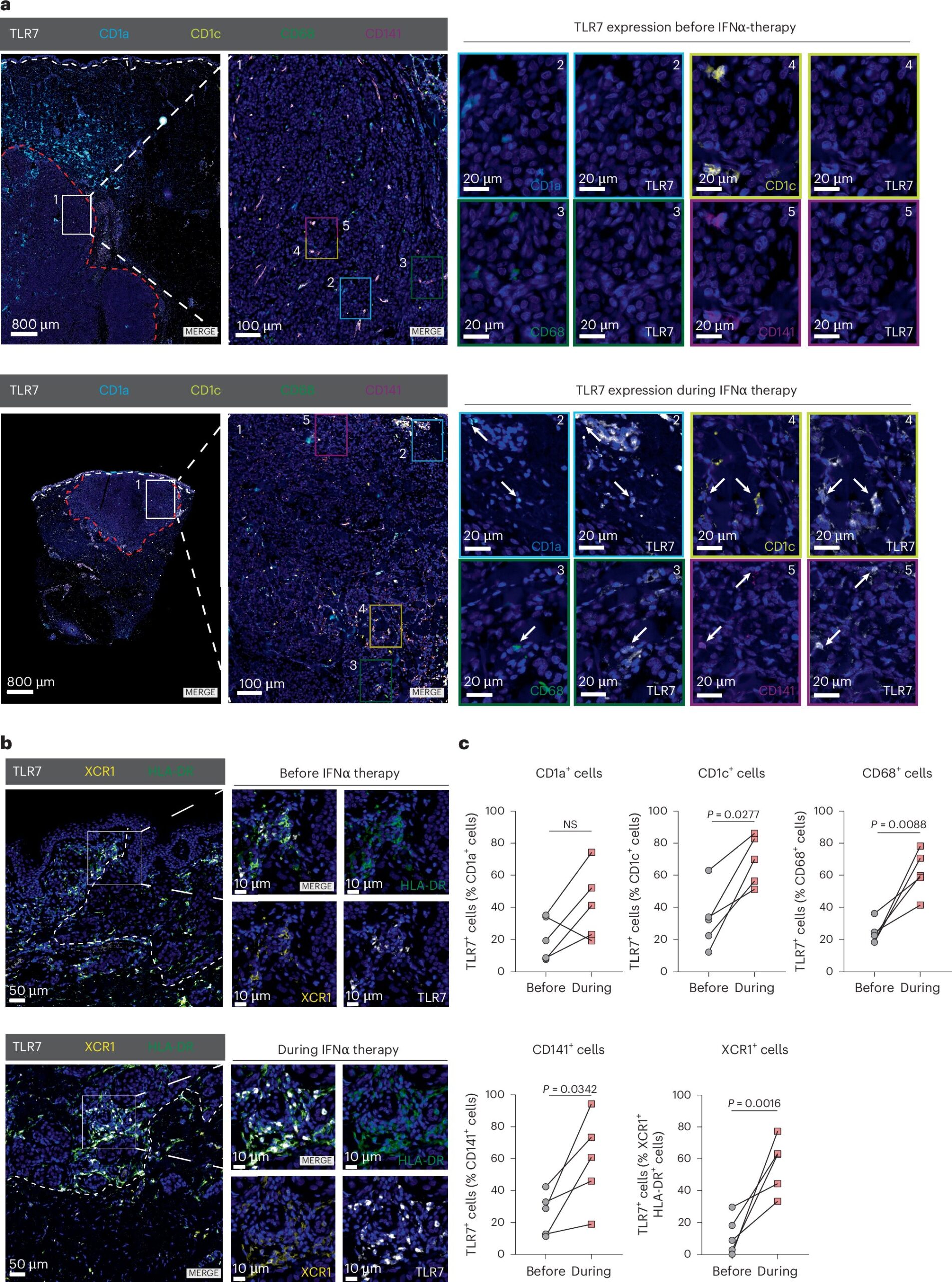
The combination therapy under investigation involves the systemic administration of IFN-I, a tissue hormone that plays an important role in regulating immune responses, alongside a local application of Imiquimod, which is already FDA-approved for the treatment of basal cell carcinomas. Imiquimod works by activating the TLR7/8 receptors, which trigger an immune response, particularly through the activation of plasmacytoid dendritic cells (pDCs), important components of the immune system.
In a groundbreaking discovery, the researchers found that when Imiquimod is applied topically to tumors, pDCs are activated, resulting in the production of IFN-I. This provides a two-pronged approach that not only enhances the immune response directly at the site of the tumor, but also primes the rest of the immune system to target distant cancer cells, including metastatic sites.
How the Therapy Works
The study demonstrated the powerful synergistic effect between systemic IFN-I and topical Imiquimod in both local and metastatic treatment. The key immune players involved in this combination are pDCs, dendritic cells, and macrophages. When Imiquimod activates pDCs, they release IFN-I, which in turn sensitizes other immune cells within the tumor microenvironment. This process leads to:
- Cancer Cell Death at the Treated Tumor Site: Imiquimod’s activation of the immune system inhibits the formation of new blood vessels (a process known as angiogenesis) at the tumor site via the cytokine IL12. This contributes to tumor cell death at the treated sites.
- Increased Immune Response to Distant Metastases: The combination therapy does more than just attack the primary tumor. By activating immune pathways at the primary tumor site, the therapy also mobilizes the immune system to target distant metastases, effectively preventing the formation of new metastatic tumors and reducing the potential for tumor relapse.
The results point to a significant breakthrough not only in treating locally accessible tumors, like melanoma and breast cancer, but also in tackling the challenge of metastasis, one of the most daunting aspects of cancer treatment.
Promising Results in Mouse Tumor Models
The research team conducted extensive preclinical tests using mouse models of melanoma and breast cancer—both of which are well-established models for testing immune-based cancer therapies. Both cancers are known for their propensity to metastasize and, in many cases, are treated with local therapies. These models provided a rigorous framework to test the novel therapy’s effects.
The combination of systemic interferon therapy (IFN-I) and local imiquimod application yielded promising results. The therapy caused tumor cells to die at the primary site while simultaneously inducing a powerful immune response capable of reducing the spread of metastatic tumors. This dual action highlights the potential of this combined approach in managing both localized and metastatic cancer.
This combination immunotherapy thus appears to hold promise as a therapeutic strategy not only for treating the primary tumor but also for fighting residual or distant metastases, something that is often the cause of relapse in cancer patients.
Implications for Melanoma and Breast Cancer Treatment
Melanoma and breast cancer are among the most studied and treatable cancers, but their high potential for metastasis makes them a challenge in many cases. The combination therapy investigated by Sibilia and her team showed significant effects on both the locally treated tumors and distant metastases, which are particularly difficult to manage with conventional treatments. For melanoma, which is often diagnosed in its advanced stages, and breast cancer, with its frequent recurrence and spread to other parts of the body, this new therapy has the potential to significantly improve patient outcomes.
Moreover, the findings hold promise for checkpoint inhibitors, such as those used in immune checkpoint blockade therapies, which are designed to unleash a patient’s immune system to attack cancer cells. The new therapy was shown to increase melanoma sensitivity to checkpoint inhibitors, potentially improving the effectiveness of these widely used treatments.
Dr. Philipp Novoszel, a researcher at MedUni Vienna and one of the study’s first authors, emphasized the importance of local Imiquimod treatment as a critical factor in ensuring the effectiveness of systemic IFN-I therapy in clearing distant metastases. As he pointed out, this topical treatment is essential in creating a tumor-friendly environment that allows the immune system to successfully fight cancer at both the site of the tumor and other distant sites of metastasis.
Next Steps and Future Directions
The combination therapy, though still in its preclinical phase, offers a glimpse into the future of cancer treatment. While the results are promising, there are still several steps required before this therapy can be tested in clinical settings. As highlighted by Dr. Martina Sanlorenzo, dermato-oncologist at MedUni Vienna and a co-first author of the study, the well-known safety profile of systemic interferon and Imiquimod’s proven effects on dendritic cell activation suggest that this combination therapy could potentially translate into tangible effects for patients with metastatic melanoma or breast cancer.
The research team hopes to continue refining this immunotherapeutic strategy to ensure it maximizes its potential for patients who are either not responding well to existing therapies or whose cancers have proven resistant to traditional treatments. By developing combined treatment options that leverage both immune system activation and local therapies, Sibilia and her team aim to improve long-term prognosis for patients battling some of the most challenging forms of cancer.
Conclusion
With new findings published in Nature Cancer, the combination of interferon-I and Imiquimod presents a hopeful step forward in the treatment of melanoma, breast cancer, and potentially other cancers prone to metastasis. By combining local immune response activation with systemic treatments, this novel approach not only promises better tumor control at the local site but also offers an immune boost that could suppress the spread of distant metastases, a major concern in advanced cancers.
As the team at the Medical University of Vienna continues to explore this innovative therapy, the medical world may be on the cusp of expanded immunotherapy options that offer more effective outcomes for cancer patients, particularly those suffering from hard-to-treat cancers like melanoma and breast cancer. This research not only paves the way for enhanced cancer treatment but also shows the power of combining established therapies in new and creative ways to maximize their potential.
Reference: Systemic IFN-I combined with topical TLR7/8 agonists promotes distant tumor suppression by c-Jun-dependent IL-12 expression in dendritic cells, Nature Cancer (2025). DOI: 10.1038/s43018-024-00889-9, www.nature.com/articles/s43018-024-00889-9
Behind every word on this website is a team pouring heart and soul into bringing you real, unbiased science—without the backing of big corporations, without financial support.
When you share, you’re doing more than spreading knowledge.
You’re standing for truth in a world full of noise. You’re empowering discovery. You’re lifting up independent voices that refuse to be silenced.
If this story touched you, don’t keep it to yourself.
Share it. Because the truth matters. Because progress matters. Because together, we can make a difference.
Your share is more than just a click—it’s a way to help us keep going.