In the microscopic depths of our intestines, a silent war rages—a battle that’s been going on for billions of years. On one side are bacteria, the microbial architects of our inner ecosystems. On the other: bacteriophages—viruses with a singular obsession—hijacking and killing bacteria with ruthless efficiency. This battle has long remained invisible, elusive, and mysterious—until now.
Scientists at the University of California, Irvine have unveiled a remarkable innovation: a live-imaging system called Phollow, a technological marvel capable of tracking bacteriophages as they infiltrate, replicate, and propagate through the guts of zebrafish at single-virion resolution. With Phollow, researchers are finally able to visualize, in real time, the elusive transmission dynamics of these microbial predators—and the implications are staggering.
This breakthrough, published in Nature Microbiology, is not just a technical feat—it’s a leap into an unseen world. And it could reshape how we understand our microbiome, engineer future medical treatments, and perhaps even rewrite the rules of microbial ecology.
The Phantom Virus Hunters of the Gut
Bacteriophages—or simply phages—are the most abundant biological entities on Earth. For every bacterium, there are likely ten phages. Though invisible to the naked eye, their reach is cosmic. These nanoscopic entities drift through oceans, soil, and even the atmosphere—but perhaps nowhere are they more intimate than in the human gut.
Colonizing our intestines shortly after birth, phages play paradoxical roles. Sometimes they act as peacekeepers, regulating bacterial populations and maintaining microbial balance. Other times, they’re saboteurs—spurring chaos by promoting horizontal gene transfer or activating latent pathogenic traits in bacteria. They’re not passive residents; they are players in the grand drama of our internal ecosystem.
But understanding how phages move, replicate, and affect their bacterial hosts in a real-world biological setting has always been a puzzle. Traditional approaches only offered fragmented glimpses—limited by static imaging, oversimplified in vitro models, or culture-dependent techniques. What was missing was a contextualized, high-resolution, real-time window into the life of a phage inside a living animal.
Phollow: Turning Phage Science Inside-Out
To address this, researchers at UC Irvine built Phollow, a dazzling suite of live-imaging technologies capable of watching phages spread inside a living gut. The innovation hinges on a mix of molecular engineering, advanced microscopy, and a clever use of germ-free zebrafish—a transparent vertebrate model ideal for visualizing internal dynamics in real-time.
Using engineered strains of E. coli and Plesiomonas as bacterial hosts, the scientists created a stable microbial environment in the zebrafish gut. Then, they introduced fluorescently tagged bacteriophages, effectively lighting up the invading viruses like neon signs. These engineered hosts, referred to as Phollow virocells, allowed researchers to pinpoint when and where viral replication took place.
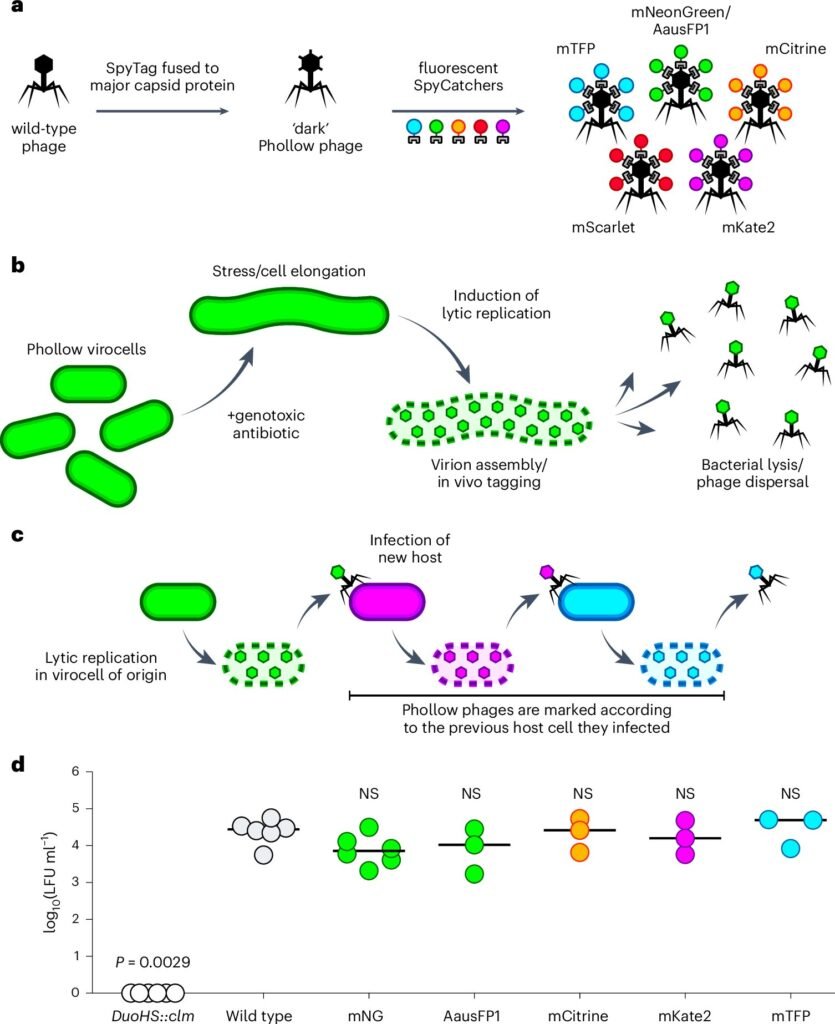
To trigger outbreaks, researchers applied antibiotics like mitomycin C, known to induce viral replication in temperate phages. Time-lapse imaging, super-resolution microscopy, flow virometry, and expansion microscopy allowed the team to observe the ensuing viral replication and dispersal—literally capturing phages as they exploded from lysed bacteria and flooded the zebrafish gut.
From Lysis to Launch: The Phage Outbreak in Action
The imaging revealed something remarkable. Upon induction, phages inside virocells formed dense viral aggregates, which—upon bacterial lysis—burst into diffuse clouds of rapidly moving particles. These viral clouds spread swiftly throughout the gut, their movement shaped by local fluid dynamics and tissue architecture.
The peak of viral replication occurred one hour after induction, involving around 20% of the bacterial population. Researchers could now count the number of viral foci—on average, about 1.6 per micron of bacterial cell length—and even measure their surface area. Using three-dimensional projections, they found that some foci were about 100 times larger than a single phage capsid—massive viral clusters ready to unleash a second infection wave.
Even more fascinating was the systemic journey of the phages. While E. coli-derived phages stayed mostly localized, Plesiomonas-derived phages made it to distant organs like the liver and brain, indicating a capacity for systemic spread. This translocation hints at the complex ways in which phages might affect host biology beyond the gut—raising both therapeutic opportunities and safety concerns.
Antibiotics as Viral Accelerants
Antibiotics aren’t just bacterial killers—they’re also phage whisperers. In this study, researchers used mitomycin C, ciprofloxacin, and trimethoprim to stimulate phage replication. Surprisingly, all three drugs induced comparable levels of virion output.
In zebrafish treated with trimethoprim, the gut was overwhelmed with phages within four hours, with the infectious clouds largely gone by 24 hours. Yet the viruses weren’t gone entirely. While they disappeared from tissues, they lingered in the surrounding water, suggesting that phages persist in the environment after release—possibly seeking new hosts or lying dormant for future outbreaks.
Such insights may change how we think about antibiotic use. Not only do these drugs affect bacterial populations—they may also act as chemical cues that trigger viral outbreaks, with unpredictable ripple effects in microbial ecosystems.
Catching a Second Wind: Proof of Phage Transmission
One of the most striking findings was the direct evidence of horizontal transmission—the spread of phages from one bacterial host to another. In vitro, Phollow phages clearly infected new host cells, confirming multiple rounds of replication. But in vivo, the evidence was even more compelling. After the initial outbreak, researchers observed a second wave of phage replication, confirming that virions released from one cell had successfully infected and replicated in another.
This capability—to monitor onward transmission inside a living organism—is a game-changer. It opens the door to understanding how phage epidemics unfold, how containment might work, and how engineered phage therapies could be fine-tuned for precision targeting.
A Window into the Future of Microbiome Medicine
Phollow is not just a research tool—it’s a prototype for the future of live microbial diagnostics. With its ability to track phages across space and time, Phollow represents a multiscale, multidimensional platform for investigating microbial and viral dynamics.
Imagine engineering beneficial phages that spread through the gut to suppress harmful bacteria, deliver gene therapies, or modulate the immune system. Imagine targeting disease-causing bacteria with tailored phage cocktails that self-propagate—displacing pathogens without harming the rest of the microbiome.
But for such dreams to become reality, understanding transmission dynamics is key. Phollow provides exactly that—contextual data on where, when, and how phages replicate. It shows whether virions are floating freely, embedded in tissues, or still latent within bacterial genomes as prophages. This level of insight is crucial if we’re to wield phages with surgical precision.
A New Lens on Microbial Warfare
What makes Phollow exceptional isn’t just the elegant imaging—it’s the conceptual shift it brings. For the first time, we’re not just studying phages in petri dishes or dissecting dead tissue samples. We’re watching the battle unfold in real time, in living, breathing organisms.
This turns phage biology into an active discipline—a visual science where behavior, context, and kinetics matter as much as genetics or molecular structure. It brings together fields as diverse as synthetic biology, virology, microbial ecology, imaging technology, and therapeutic engineering.
And while zebrafish are only a model system, the principles learned here could extend to humans. Our own guts teem with billions of phages, silently shaping our health, resilience, and disease susceptibility. With tools like Phollow, we may soon be able to read the viral signatures of these invisible forces—and perhaps learn to direct them.
Conclusion: The Promise of Phollow
In a world grappling with antibiotic resistance, emerging pathogens, and a renewed focus on the human microbiome, bacteriophages are gaining renewed attention as allies in medicine. But using them responsibly and effectively requires knowing how they behave in complex ecosystems. Phollow offers a remarkable new lens—a way to trace the life of a single virion across time and space, inside a living host.
The team at UC Irvine hasn’t just built a microscope. They’ve built a bridge—between invisible biology and human understanding, between curiosity and control.
The gut may seem like a quiet place, but with Phollow, we now know better. There is a war happening. And for the first time, we’re watching it live.
Reference: Lizett Ortiz de Ora et al, Phollow reveals in situ phage transmission dynamics in the zebrafish gut microbiome at single-virion resolution, Nature Microbiology (2025). DOI: 10.1038/s41564-025-01981-1
Behind every word on this website is a team pouring heart and soul into bringing you real, unbiased science—without the backing of big corporations, without financial support.
When you share, you’re doing more than spreading knowledge.
You’re standing for truth in a world full of noise. You’re empowering discovery. You’re lifting up independent voices that refuse to be silenced.
If this story touched you, don’t keep it to yourself.
Share it. Because the truth matters. Because progress matters. Because together, we can make a difference.
Your share is more than just a click—it’s a way to help us keep going.
