In the ancient depths of Earth’s biological history, something extraordinary happened—something so foundational, so transformative, that it reshaped the very architecture of life. A recent study published in the Proceedings of the National Academy of Sciences (PNAS), the result of an ambitious international collaboration, offers a fascinating window into this pivotal moment: the birth of the eukaryotic cell. A new kind of life—complex, organized, and full of evolutionary potential—was born from a critical evolutionary phase transition, one that scientists are now comparing to phenomena in quantum physics and computer science.
The Eukaryotic Enigma: The Black Hole of Biology
The emergence of eukaryotic cells—those that make up everything from mushrooms to whales to humans—marks the single greatest leap in complexity in Earth’s 4.5-billion-year evolutionary saga. These cells, unlike their simpler prokaryotic ancestors, boast intricate internal structures: nuclei, mitochondria, and various organelles functioning like specialized compartments in a high-efficiency factory.
But despite decades of research and the acceptance of the endosymbiotic theory—which posits that eukaryotic cells arose from a symbiotic fusion between an ancient archaeon and a bacterium—one immense challenge has remained. There’s a gaping chasm in the fossil and genetic record. Scientists call it “the black hole at the heart of biology”—the absence of clear evolutionary intermediates leading up to the eukaryotic cell.
Now, thanks to a trailblazing collaboration among researchers from Johannes Gutenberg University Mainz (Germany), the University of Valencia (Spain), the Polytechnic University of Madrid (Spain), and the University of Zurich (Switzerland), this black hole is being illuminated. Their research brings together physics, computational modeling, and evolutionary biology in a dazzlingly original way—and offers new answers.
Proteins, Genes, and the Mathematical Order of Life
At the heart of this research lies a deceptively simple question: How did genetic architecture evolve to support the vast complexity of eukaryotic life?
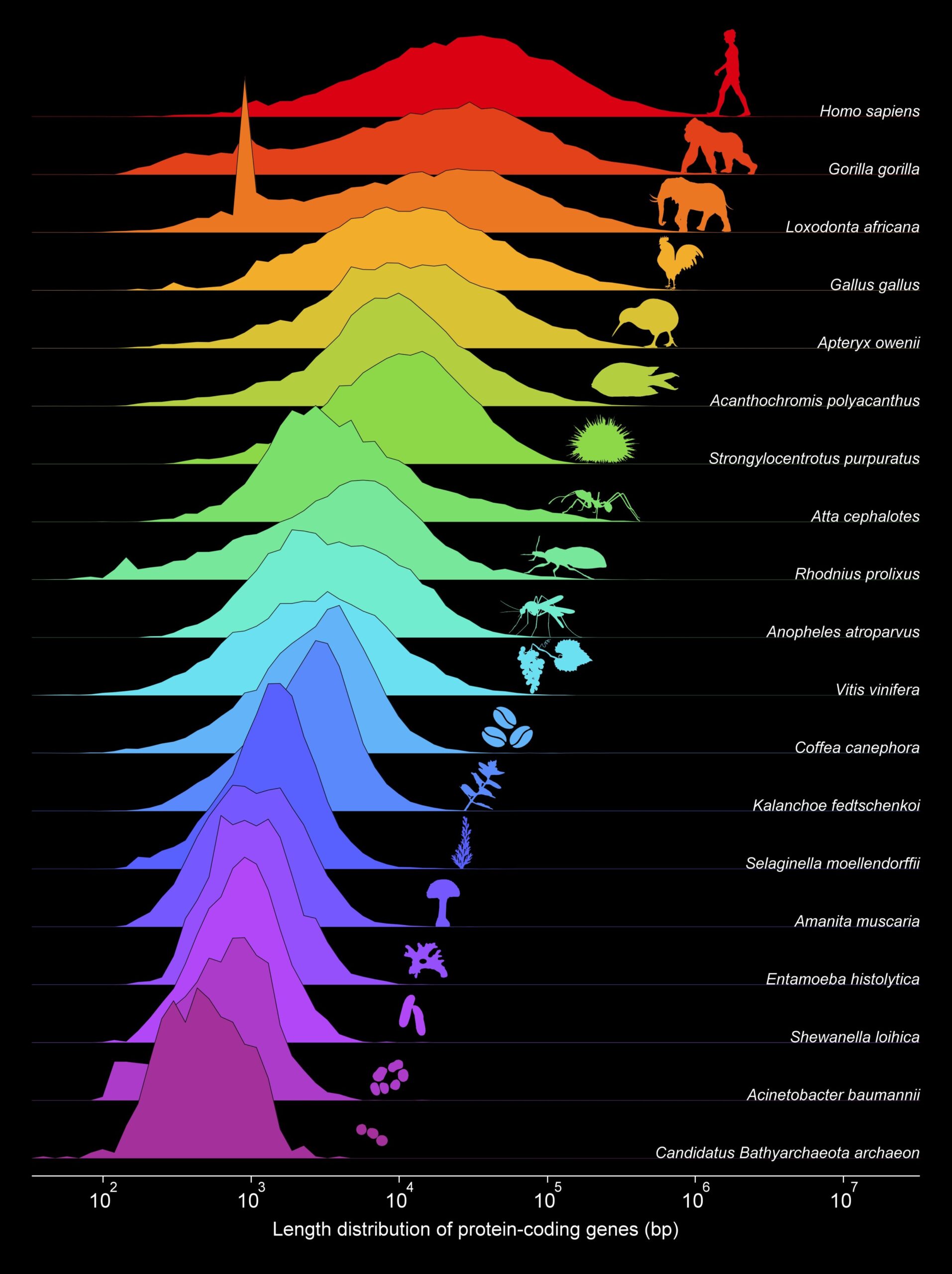
The team began by examining proteins—the molecular machines that carry out nearly every function within a cell—and the genes that code for them. Using a colossal dataset of 9,913 proteomes (entire sets of proteins from organisms) and 33,627 genomes, the researchers found a pattern: both protein lengths and gene lengths across all domains of life follow what’s called a log-normal distribution. This statistical pattern is common in systems governed by multiplicative processes—where elements grow by multiplying rather than adding.
But what does that mean for life?
Dr. Enrique M. Muro, representing Johannes Gutenberg University Mainz, explained that by modeling gene length evolution as a stochastic (random) multiplicative process, the team could trace how genes—and the complexity they enable—have grown over time. From the earliest hypothetical ancestor known as LUCA (the Last Universal Common Ancestor), the average gene length has grown exponentially. And intriguingly, so has the potential for organismal complexity.
Scaling Complexity: From Simplicity to Sophistication
One of the most striking findings was the relationship between gene length and protein length. In prokaryotic organisms (bacteria and archaea), where genes are densely packed with little to no “junk” or non-coding DNA, gene length and protein length grew together in lockstep. But that changes at a specific threshold: when the average gene length crosses about 1,500 nucleotides, something dramatic happens.
The protein lengths stop growing.
Instead, they plateau at an average of about 500 amino acids, while the genes continue to stretch. Why? Because from this point forward, evolution started inserting more non-coding sequences—regions of DNA that don’t directly code for proteins but play regulatory or structural roles.
This moment, the researchers argue, marks the emergence of the eukaryotic cell. It’s the evolutionary watershed, a turning point that divided life into two epochs: the coding-dominated phase of prokaryotes, and the non-coding-rich phase of eukaryotes.
A Critical Phase Transition: Borrowed from Physics
To explain this leap, the researchers turned to an idea from physics: phase transitions—those sudden shifts in state that happen in systems under changing conditions, like water freezing into ice or iron becoming magnetized.
They discovered that the evolutionary trajectory of gene lengths behaves much like a system undergoing a phase transition. There’s even evidence of critical slowing down, a known feature of physical phase changes where a system lingers near the transition point, stuck in metastable states.
“This is corroborated in early protists and fungi,” noted Dr. Fernando Ballesteros of the University of Valencia, referring to primitive eukaryotes that seemed to hover on the brink of complexity for extended evolutionary periods.
The research team identifies a specific critical point: around 2.6 billion years ago. At this time, the average gene length crossed the 1,500-nucleotide threshold, and evolution underwent an algorithmic phase shift.
From Computation to Creation: The Algorithmic Shift
But what does “algorithmic” mean in this context?
Professor Jordi Bascompte of the University of Zurich explains it beautifully. In early life, increasing protein complexity was a relatively simple computational problem. With short genes and proteins, evolution could “search” the space of possible sequences efficiently. But as proteins grew longer and more intricate, that search became exponentially harder—a computational nightmare.
The solution?
Add non-coding sequences. These regulatory regions, introns, and other genetic elements created modularity. They allowed for alternative splicing, regulation, and massive information compression. With the development of the nucleus and spliceosome, eukaryotic cells gained the ability to “edit” and “repackage” genetic information with unprecedented flexibility. Evolution, in a sense, rewrote its algorithm to handle more complex instructions more efficiently.
Thus, the eukaryotic cell wasn’t just a new kind of organism—it was a new kind of information processor.
Beyond the Cell: The Ripple Effect of Complexity
Once this barrier was breached, evolution accelerated in astonishing ways. The rise of eukaryotic cells set the stage for other major transitions: multicellularity, sexual reproduction, cellular differentiation, and eventually, the emergence of consciousness. Each of these leaps was enabled by the foundational change in how genetic information was structured and interpreted.
“The phase transition to eukaryotic cells was the mother of all evolutionary innovations,” said Dr. Muro. “It provided the informational and energetic architecture required to build everything that followed.”
Indeed, this transition wasn’t just a one-time event—it was the trigger for cascading waves of complexity that reshaped the biosphere and, ultimately, led to us.
Interdisciplinary Brilliance: Biology Meets Physics and Beyond
What makes this study particularly compelling is its elegant interdisciplinarity. By blending evolutionary theory, statistical modeling, computational biology, and the mathematics of critical phenomena, the team has laid down a new framework for understanding biological complexity.
“Biology has always needed a theory of complexity,” said Dr. Bartolo Luque of the Polytechnic University of Madrid. “This is a step toward that goal.”
And it’s not just for evolutionary biologists. The implications stretch into areas like information theory, artificial intelligence, and even the search for life beyond Earth. If life elsewhere also undergoes such phase transitions in complexity, we may someday recognize echoes of our own evolutionary story written in alien biochemistries.
Conclusion: The Dawn of a New Understanding
In peeling back the layers of the evolutionary record, this groundbreaking study has illuminated a pivotal truth: the rise of the eukaryotic cell was not merely an event—it was a transformation in the very fabric of life’s computational and energetic infrastructure. A phase transition, both physical and algorithmic, that opened the door to complexity, consciousness, and everything we hold dear.
As science continues to explore the profound intersections of biology, mathematics, and information theory, we may yet discover deeper laws governing the evolution of life—not as a random walk through chaos, but as a journey shaped by universal principles, elegant patterns, and critical transitions.
The black hole at the heart of biology is no longer so dark. It is a window—a brilliant, blazing insight into how life became not just possible, but profound.
Reference: Enrique M. Muro et al, The emergence of eukaryotes as an evolutionary algorithmic phase transition, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2422968122